TGF-β controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription in mice
- PMID: 38488000
- PMCID: PMC10947970
- DOI: 10.1172/JCI172095
TGF-β controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription in mice
Abstract
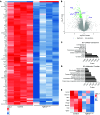
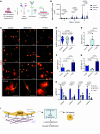
Premature birth disrupts normal lung development and places infants at risk for bronchopulmonary dysplasia (BPD), a disease disrupting lung health throughout the life of an individual and that is increasing in incidence. The TGF-β superfamily has been implicated in BPD pathogenesis, however, what cell lineage it impacts remains unclear. We show that TGFbr2 is critical for alveolar epithelial (AT1) cell fate maintenance and function. Loss of TGFbr2 in AT1 cells during late lung development leads to AT1-AT2 cell reprogramming and altered pulmonary architecture, which persists into adulthood. Restriction of fetal lung stretch and associated AT1 cell spreading through a model of oligohydramnios enhances AT1-AT2 reprogramming. Transcriptomic and proteomic analyses reveal the necessity of TGFbr2 expression in AT1 cells for extracellular matrix production. Moreover, TGF-β signaling regulates integrin transcription to alter AT1 cell morphology, which further impacts ECM expression through changes in mechanotransduction. These data reveal the cell intrinsic necessity of TGF-β signaling in maintaining AT1 cell fate and reveal this cell lineage as a major orchestrator of the alveolar matrisome.
Keywords: Extracellular matrix; Integrins; Pulmonology; Respiration.
Conflict of interest statement
Figures





Update of
-
TGFβ controls alveolar type 1 epithelial cell plasticity and alveolar matrisome gene transcription.bioRxiv [Preprint]. 2023 May 10:2023.05.09.540035. doi: 10.1101/2023.05.09.540035. bioRxiv. 2023. Update in: J Clin Invest. 2024 Jan 11;134(6):e172095. doi: 10.1172/JCI172095. PMID: 37214932 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

