The cholesterol biosynthesis enzyme FAXDC2 couples Wnt/β-catenin to RTK/MAPK signaling
- PMID: 38488003
- PMCID: PMC10940096
- DOI: 10.1172/JCI171222
The cholesterol biosynthesis enzyme FAXDC2 couples Wnt/β-catenin to RTK/MAPK signaling
Abstract
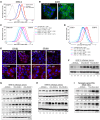
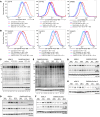
Wnts, cholesterol, and MAPK signaling are essential for development and adult homeostasis. Here, we report that fatty acid hydroxylase domain containing 2 (FAXDC2), a previously uncharacterized enzyme, functions as a methyl sterol oxidase catalyzing C4 demethylation in the Kandutsch-Russell branch of the cholesterol biosynthesis pathway. FAXDC2, a paralog of MSMO1, regulated the abundance of the specific C4-methyl sterols lophenol and dihydro-T-MAS. Highlighting its clinical relevance, FAXDC2 was repressed in Wnt/β-catenin-high cancer xenografts, in a mouse genetic model of Wnt activation, and in human colorectal cancers. Moreover, in primary human colorectal cancers, the sterol lophenol, regulated by FAXDC2, accumulated in the cancerous tissues and not in adjacent normal tissues. FAXDC2 linked Wnts to RTK/MAPK signaling. Wnt inhibition drove increased recycling of RTKs and activation of the MAPK pathway, and this required FAXDC2. Blocking Wnt signaling in Wnt-high cancers caused both differentiation and senescence; and this was prevented by knockout of FAXDC2. Our data show the integration of 3 ancient pathways, Wnts, cholesterol synthesis, and RTK/MAPK signaling, in cellular proliferation and differentiation.
Keywords: Cancer; Cholesterol; Metabolism; Oncology; Phosphotyrosine.
Conflict of interest statement
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

