Prelimbic cortical pyramidal neurons to ventral tegmental area projections promotes arousal from sevoflurane anesthesia
- PMID: 38488453
- PMCID: PMC10941502
- DOI: 10.1111/cns.14675
Prelimbic cortical pyramidal neurons to ventral tegmental area projections promotes arousal from sevoflurane anesthesia
Abstract
Aims: General anesthesia has been used in surgical procedures for approximately 180 years, yet the precise mechanism of anesthetic drugs remains elusive. There is significant anatomical connectivity between the ventral tegmental area (VTA) and the prelimbic cortex (PrL). Projections from VTA dopaminergic neurons (VTADA ) to the PrL play a role in the transition from sevoflurane anesthesia to arousal. It is still uncertain whether the prelimbic cortex pyramidal neuron (PrLPyr ) and its projections to VTA (PrLPyr -VTA) are involved in anesthesia-arousal regulation.
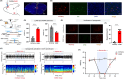
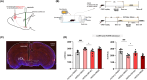
Methods: We employed chemogenetics and optogenetics to selectively manipulate neuronal activity in the PrLPyr -VTA pathway. Electroencephalography spectra and burst-suppression ratios (BSR) were used to assess the depth of anesthesia. Furthermore, the loss or recovery of the righting reflex was monitored to indicate the induction or emergence time of general anesthesia. To elucidate the receptor mechanisms in the PrLPyr -VTA projection's impact on anesthesia and arousal, we microinjected NMDA receptor antagonists (MK-801) or AMPA receptor antagonists (NBQX) into the VTA.
Results: Our findings show that chemogenetic or optogenetic activation of PrLPyr neurons prolonged anesthesia induction and promoted emergence. Additionally, chemogenetic activation of the PrLPyr -VTA neural pathway delayed anesthesia induction and promoted anesthesia emergence. Likewise, optogenetic activation of the PrLPyr -VTA projections extended the induction time and facilitated emergence from sevoflurane anesthesia. Moreover, antagonizing NMDA receptors in the VTA attenuates the delayed anesthesia induction and promotes emergence caused by activating the PrLPyr -VTA projections.
Conclusion: This study demonstrates that PrLPyr neurons and their projections to the VTA are involved in facilitating emergence from sevoflurane anesthesia, with the PrLPyr -VTA pathway exerting its effects through the activation of NMDA receptors within the VTA.
Keywords: general anesthesia; prelimbic cortex; pyramidal neuron; sevoflurane; ventral tegmental area.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors state that there are no conflicts of interest to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

