Illuminating the terminal nerve: Uncovering the link between GnRH-1 neuron and olfactory development
- PMID: 38488687
- PMCID: PMC10958589
- DOI: 10.1002/cne.25599
Illuminating the terminal nerve: Uncovering the link between GnRH-1 neuron and olfactory development
Abstract
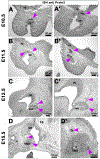
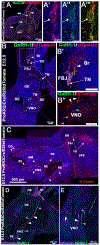
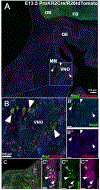
During embryonic development, the olfactory placode (OP) generates migratory neurons, including olfactory pioneer neurons, cells of the terminal nerve (TN), gonadotropin-releasing hormone-1 (GnRH-1) neurons, and other uncharacterized neurons. Pioneer neurons from the OP induce olfactory bulb (OB) morphogenesis. In mice, GnRH-1 neurons appear in the olfactory system around mid-gestation and migrate via the TN axons to different brain regions. The GnRH-1 neurons are crucial in controlling the hypothalamic-pituitary-gonadal axis. Kallmann syndrome is characterized by impaired olfactory system development, defective OBs, secretion of GnRH-1, and infertility. The precise mechanistic link between the olfactory system and GnRH-1 development remains unclear. Studies in humans and mice highlight the importance of the prokineticin-2/prokineticin-receptor-2 (Prokr2) signaling pathway in OB morphogenesis and GnRH-1 neuronal migration. Prokr2 loss-of-function mutations can cause Kallmann syndrome (KS), and hence the Prokr2 signaling pathway represents a unique model to decipher the olfactory/GnRH-1 connection. We discovered that Prokr2 is expressed in the TN neurons during the critical period of GnRH-1 neuron formation, migration, and induction of OB morphogenesis. Single-cell RNA sequencing identified that the TN is formed by neurons distinct from the olfactory neurons. The TN neurons express multiple genes associated with KS. Our study suggests that the aberrant development of pioneer/TN neurons might cause the KS spectrum.
Keywords: GnRH neurons; Kallmann syndrome; migratory mass; olfactory bulb; pioneer neurons; prokineticin receptor-2; terminal nerve.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
Figures




Update of
-
Illuminating the Terminal Nerve: Uncovering the Link between GnRH-1 and Olfactory Development.bioRxiv [Preprint]. 2023 Sep 3:2023.08.31.555770. doi: 10.1101/2023.08.31.555770. bioRxiv. 2023. Update in: J Comp Neurol. 2024 Mar;532(3):e25599. doi: 10.1002/cne.25599. PMID: 37693459 Free PMC article. Updated. Preprint.
References
-
- Afonso Lopes L, Benador D, Wacker P, Wyss M, Sizonenko PC. 1995. Turner’s syndrome and hypogonadotrophic hypogonadism: thalassemia major and hemochromatosis. J Pediatr Endocrinol Metab 8: 73–77. - PubMed
-
- Alsters SI, Goldstone AP, Buxton JL, Zekavati A, Sosinsky A, Yiorkas AM, Holder S, Klaber RE, Bridges N, van Haelst MM, le Roux CW, Walley AJ, Walters RG, Mueller M, Blakemore AI. 2015. Truncating Homozygous Mutation of Carboxypeptidase E (CPE) in a Morbidly Obese Female with Type 2 Diabetes Mellitus, Intellectual Disability and Hypogonadotrophic Hypogonadism. PLoS One 10: e0131417. - PMC - PubMed
-
- Avbelj Stefanija M, Jeanpierre M, Sykiotis GP, Young J, Quinton R, Abreu AP, Plummer L, Au MG, Balasubramanian R, Dwyer AA, Florez JC, Cheetham T, Pearce SH, Purushothaman R, Schinzel A, Pugeat M, Jacobson-Dickman EE, Ten S, Latronico AC, Gusella JF, Dode C, Crowley WF Jr., Pitteloud N. 2012. An ancient founder mutation in PROKR2 impairs human reproduction. Hum Mol Genet 21: 4314–4324. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

