Effect of Vaginal Microecological Alterations on Female Pelvic Organ Prolapse
- PMID: 38488886
- PMCID: PMC11052768
- DOI: 10.1007/s00192-024-05759-7
Effect of Vaginal Microecological Alterations on Female Pelvic Organ Prolapse
Abstract
Introduction and hypothesis: The objective was to investigate the correlation between endogenous vaginal microecological alterations and female pelvic organ prolapse (POP).
Methods: Patients who underwent vaginal hysterectomy were retrospectively analyzed as the POP group (n = 30) and the non-POP group (n = 30). The vaginal microbial metabolites and enzyme levels were tested using the dry chemoenzymatic method. The mRNA and protein expression were tested using real-time quantitative PCR and immunohistochemistry. SPSS version 25.0 and GraphPad Prism 8.0 were performed for statistical analysis.
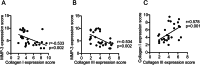
Results: Compared with the non-POP group, the vaginal pH, H2O2 positivity and leukocyte esterase positivity were higher in patients with POP (all p < 0.05). Further analysis showed that patients with pelvic organ prolapse quantification (POP-Q) stage IV had higher rates of vaginal pH, H2O2 positivity and leukocyte esterase positivity than those with POP-Q stage III. Additionally, the mRNA expression of decorin (DCN), transforming growth factor beta 1 (TGF-β1), and matrix metalloproteinase-3 (MMP-3) in uterosacral ligament tissues were higher, whereas collagen I and III were lower. Similarly, the positive expression of MMP-3 in uterosacral ligament tissue was significantly upregulated in the POP group compared with the non-POP group (p = 0.035), whereas collagen I (p = 0.004) and collagen III (p = 0.019) in uterosacral ligament tissue were significantly downregulated in the POP group. Correlation analysis revealed that there was a significant correlation between vaginal microecology and collagen metabolism. In addition, MMP-3 correlated negatively with collagen I and collagen III (p = 0.002, r = -0.533; p = 0.002, r = -0.534 respectively), whereas collagen I correlated positively with collagen III (p = 0.001, r = 0.578).
Conclusions: Vaginal microecological dysbiosis affects the occurrence of female POP, which could be considered a novel therapeutic option.
Keywords: Collagen I; Collagen III; Matrix metalloproteinase-3; Pelvic organ prolapse; Vaginal microbiome.
© 2024. The Author(s).
Conflict of interest statement
None.
Figures




References
-
- [Chinese guideline for the diagnosis and management of pelvic organ prolapse (2020 version)]. Zhonghua Fu Chan Ke Za Zhi. 2020;55(5):300–6. 10.3760/cma.j.cn112141-20200106-00016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

