Holistic, Long-Term Management of People with Relapsing Multiple Sclerosis with Cladribine Tablets: Expert Opinion from France
- PMID: 38488979
- PMCID: PMC11136930
- DOI: 10.1007/s40120-024-00589-7
Holistic, Long-Term Management of People with Relapsing Multiple Sclerosis with Cladribine Tablets: Expert Opinion from France
Abstract
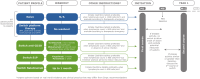
Cladribine tablets (CladT) has been available for therapeutic use in France since March 2021 for the management of highly active relapsing multiple sclerosis (RMS). This high-efficacy disease-modifying therapy (DMT) acts as an immune reconstitution therapy. In contrast to most high-efficacy DMTs, which act via continuous immunosuppression, two short courses of oral treatment with CladT at the beginning of years 1 and 2 of treatment provide long-term control of MS disease activity in responders to treatment, without the need for any further pharmacological treatment for several years. Although the labelling for CladT does not provide guidance beyond the initial treatment courses, real-world data on the therapeutic use of CladT from registries of previous clinical trial participants and patients treated in routine practice indicate that MS disease activity is controlled for a period of years beyond this time for a substantial proportion of patients. Moreover, this clinical experience has provided useful information on how to initiate and manage treatment with CladT. In this article we, a group of expert neurologists from France, provide recommendations on the initiation of CladT in DMT-naïve patients, how to switch from existing DMTs to CladT for patients with continuing MS disease activity, how to manage patients during the first 2 years of treatment and finally, how to manage patients with or without MS disease activity in years 3, 4 and beyond after initiating treatment with CladT. We believe that optimisation of the use of CladT beyond its initial courses of treatment will maximise the benefits of this treatment, especially early in the course of MS when suppression of focal inflammation in the CNS is a clinical priority to limit MS disease progression.
Keywords: Cladribine tablets; Disease-modifying therapy; Holistic management; Multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
Jonathan Ciron has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi, Roche, Celgene-BMS, Alexion, and Horizon Therapeutics. Bertrand Bourre has received consulting and lecturing fees, travel grants, and unconditional research support from Alexion, Biogen, Horizon, Janssen, Merck, Novartis, Roche, Sandoz, and Sanofi. Giovanni Castelnovo received fees for consulting and speaking from Biogen, Abbvie, Merck, Novartis, Roche, Sanofi Genzyme, Merz, Celgene BMS. Anne-Marie Guennoc has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi, Roche. Jerome De Sèze has received fees for consultancy, advisory board and clinical trials from UCB, Novartis, Biogen, Merck, Teva, Genzyme/ Sanofi, Roche, Alexion, BMS/Celegene, Janssen, Horizon Therapeutics. At the time of the expert meeting, Ali-Frederic Ben-Amor was an employee of Ares-Trading SA Eysins, Switzerland, an affiliate of Merck KGaA. Carine Savarin is an employee of Merck Santé S.A.S., Lyon, France, an affiliate of Merck KGaA. Patrick Vermersch received honoraria for contributions to meetings from Biogen, Sanofi-Genzyme, Novartis, Teva, Merck, Roche, Imcyse, AB Science and BMS-Celgene, and research support from Novartis, Sanofi-Genzyme and Merck.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

