Dynamic duo: Kir6 and SUR in KATP channel structure and function
- PMID: 38489043
- PMCID: PMC10950283
- DOI: 10.1080/19336950.2024.2327708
Dynamic duo: Kir6 and SUR in KATP channel structure and function
Abstract
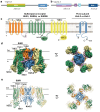
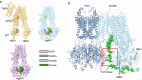
KATP channels are ligand-gated potassium channels that couple cellular energetics with membrane potential to regulate cell activity. Each channel is an eight subunit complex comprising four central pore-forming Kir6 inward rectifier potassium channel subunits surrounded by four regulatory subunits known as the sulfonylurea receptor, SUR, which confer homeostatic metabolic control of KATP gating. SUR is an ATP binding cassette (ABC) protein family homolog that lacks membrane transport activity but is essential for KATP expression and function. For more than four decades, understanding the structure-function relationship of Kir6 and SUR has remained a central objective of clinical significance. Here, we review progress in correlating the wealth of functional data in the literature with recent KATP cryoEM structures.
Keywords: ABC transporter; ATP-sensitive potassium channel; Kir6.2; cryo-EM; inward rectifier potassium channel; sulfonylurea receptor.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Production and purification of ATP-sensitive potassium channel particles for cryo-electron microscopy.Methods Enzymol. 2021;653:121-150. doi: 10.1016/bs.mie.2021.02.008. Epub 2021 Mar 22. Methods Enzymol. 2021. PMID: 34099169 Free PMC article.
-
Remodelling of the SUR-Kir6.2 interface of the KATP channel upon ATP binding revealed by the conformational blocker rhodamine 123.J Physiol. 2007 Jul 1;582(Pt 1):27-39. doi: 10.1113/jphysiol.2007.134288. Epub 2007 May 17. J Physiol. 2007. PMID: 17510180 Free PMC article.
-
Structure of an open KATP channel reveals tandem PIP2 binding sites mediating the Kir6.2 and SUR1 regulatory interface.Nat Commun. 2024 Mar 20;15(1):2502. doi: 10.1038/s41467-024-46751-5. Nat Commun. 2024. PMID: 38509107 Free PMC article.
-
Toward understanding the assembly and structure of KATP channels.Physiol Rev. 1998 Jan;78(1):227-45. doi: 10.1152/physrev.1998.78.1.227. Physiol Rev. 1998. PMID: 9457174 Review.
-
Mechanistic insights on KATP channel regulation from cryo-EM structures.J Gen Physiol. 2023 Jan 2;155(1):e202113046. doi: 10.1085/jgp.202113046. Epub 2022 Nov 28. J Gen Physiol. 2023. PMID: 36441147 Free PMC article. Review.
Cited by
-
Repositioning pinacidil and its anticonvulsant and anxiolytic properties in murine models.Sci Rep. 2024 Sep 30;14(1):22695. doi: 10.1038/s41598-024-73720-1. Sci Rep. 2024. PMID: 39349563 Free PMC article.
-
LRRC8 channel complexes counterbalance KATP channels to mediate swell-secretion coupling in mouse pancreatic β cells.JCI Insight. 2025 Apr 29;10(11):e188020. doi: 10.1172/jci.insight.188020. eCollection 2025 Jun 9. JCI Insight. 2025. PMID: 40299635 Free PMC article.
-
KATP channels and cardioprotection.Arh Farm (Belgr). 2024;74(5):625-657. doi: 10.5937/arhfarm74-51604. Epub 2024 Nov 1. Arh Farm (Belgr). 2024. PMID: 40400696
-
Expression of SUR1 isoforms in the brain and heart after ischemia/reperfusion.Front Mol Neurosci. 2025 Apr 17;18:1536409. doi: 10.3389/fnmol.2025.1536409. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40313402 Free PMC article.
-
Sodium, Potassium-Adenosine Triphosphatase as a Potential Target of the Anti-Tuberculosis Agents, Clofazimine and Bedaquiline.Int J Mol Sci. 2024 Dec 4;25(23):13022. doi: 10.3390/ijms252313022. Int J Mol Sci. 2024. PMID: 39684733 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
