Sustained Delivery of Olopatadine from Vitamin-E Loaded Contact Lenses
- PMID: 38489059
- PMCID: PMC11265619
- DOI: 10.1089/jop.2023.0111
Sustained Delivery of Olopatadine from Vitamin-E Loaded Contact Lenses
Abstract
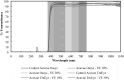
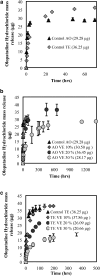
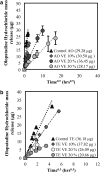
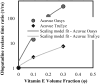
Purpose: Topical antihistamines, such as olopatadine hydrochloride, an H1 receptor antagonist, are commonly prescribed for treating allergic conjunctivitis. Drug delivery via eye drops has many deficiencies including a short residence time due to tear drainage via the nasolacrimal duct, which results in a low bioavailability and potential for side effects. These deficiencies could be mitigated by a drug-eluting contact lens such as the recently approved ACUVUE® THERAVISION™ WITH KETOTIFEN which is a daily disposable etafilcon, a drug-eluting contact lens with ketotifen (19 μg per lens). Here, we investigate the feasibility of designing a drug-eluting lens with sustained release of olopatadine for treating allergies using an extended wear lens. Methods: Nanobarrier depots composed of vitamin-E (VE) are formed through direct entrapment by ethanol-driven swelling. The drug-loaded lenses are characterized for transparency and water content. In vitro release is measured under sink conditions and fitted to a diffusion control release model to determine diffusivity and partition coefficient. Results: In vitro studies indicate that ACUVUE OASYS® and ACUVUE TruEye™ lenses loaded with ∼0.3 g of VE/g of hydrogel effectively prolong olopatadine dynamics by 7-fold and 375-fold, respectively. Incorporation of VE into the lenses retains visible light transmission and other properties. Conclusion: The VE incorporation in commercial lenses significantly increases the release duration offering the possibility of antiallergy extended wear lenses.
Keywords: bioavailability; conjunctivitis; olopatadine hydrochloride; vitamin E.
Figures
Similar articles
-
Novel Polyvinyl Pyrrolidone-Loaded Olopatadine HCl-Laden Doughnut Contact Lens to Treat Allergic Conjunctivitis.J Pharm Sci. 2020 May;109(5):1714-1724. doi: 10.1016/j.xphs.2020.01.022. Epub 2020 Jan 31. J Pharm Sci. 2020. PMID: 32007507
-
In vitro drug release and in vivo safety of vitamin E and cysteamine loaded contact lenses.Int J Pharm. 2018 Jun 15;544(2):380-391. doi: 10.1016/j.ijpharm.2017.11.059. Epub 2017 Dec 5. Int J Pharm. 2018. PMID: 29217475
-
Effect of vitamin-E integration on delivery of prostaglandin analogs from therapeutic lenses.J Colloid Interface Sci. 2019 Mar 15;539:457-467. doi: 10.1016/j.jcis.2018.12.036. Epub 2018 Dec 17. J Colloid Interface Sci. 2019. PMID: 30611041
-
Commercialization challenges for drug eluting contact lenses.Expert Opin Drug Deliv. 2020 Aug;17(8):1133-1149. doi: 10.1080/17425247.2020.1787983. Epub 2020 Jul 2. Expert Opin Drug Deliv. 2020. PMID: 32602822 Review.
-
Mechanistic modeling of ophthalmic drug delivery to the anterior chamber by eye drops and contact lenses.Adv Colloid Interface Sci. 2016 Jul;233:139-154. doi: 10.1016/j.cis.2015.08.002. Epub 2015 Aug 14. Adv Colloid Interface Sci. 2016. PMID: 26318359 Review.
Cited by
-
Dry Eye Disease: Oxidative Stress on Ocular Surface and Cutting-Edge Antioxidants.Glob Chall. 2025 May 14;9(7):e00068. doi: 10.1002/gch2.202500068. eCollection 2025 Jul. Glob Chall. 2025. PMID: 40656216 Free PMC article. Review.
References
-
- Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge: A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol 1990;108(1):84–88. - PubMed
-
- Abelson MB, Schaefer K.. Conjunctivitis of allergic origin: Immunologic mechanisms and current approaches to therapy. Surv Ophthalmol 1993;38 Suppl:115–132. - PubMed
-
- Allansmith MR, Ross RN. Ocular allergy and mast cell stabilizers. Surv Ophthalmol 1986;30(4):229–244. - PubMed
-
- Bielory L. Allergic conjunctivitis and the impact of allergic rhinitis. Curr Allergy Asthma Rep 2010;10(2):122–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

