Critical Examination of Modeling Approaches Used in Economic Evaluations of First-Line Treatments for Locally Advanced or Metastatic Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutations: A Systematic Literature Review
- PMID: 38489077
- PMCID: PMC11039500
- DOI: 10.1007/s40273-024-01362-2
Critical Examination of Modeling Approaches Used in Economic Evaluations of First-Line Treatments for Locally Advanced or Metastatic Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutations: A Systematic Literature Review
Abstract
Background: Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, with up to 32% of patients with NSCLC harboring an epidermal growth factor receptor (EGFR) mutation. NSCLC harboring an EGFR mutation has a dedicated treatment pathway, with EGFR tyrosine kinase inhibitors and platinum-based chemotherapy often being the therapy of choice.
Objective: The aim of this study was to systemically review and summarize economic models of first-line treatments used for locally advanced or metastatic NSCLC harboring EGFR mutations, as well as to identify areas for improvement for future models.
Methods: Literature searches were conducted via Ovid in PubMed, MEDLINE, MEDLINE In-Process, Embase, Evidence-Based Medicine Reviews: Health Technology Assessment, Evidence-Based Medicine Reviews: National Health Service Economic Evaluation Database, and EconLit. An initial search was conducted on 19 December 2022 and updated on 11 April 2023. Studies were selected according to predefined criteria using the Population, Intervention, Comparator, Outcome and Study design (PICOS) framework.
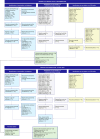
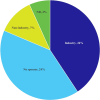
Results: Sixty-seven articles were included in the review, representing 59 unique studies. The majority of included models were cost-utility analyses (n = 52), with the remaining studies being cost-effectiveness analyses (n = 4) and a cost-minimization analysis (n = 1). Two studies incorporated both a cost-utility and cost-minimization analysis. Although the model structure across studies was consistently reported, justification for this choice was often lacking.
Conclusions: Although the reporting of economic models in NSCLC harboring EGFR mutations is generally good, many of these studies lacked sufficient reporting of justification for structural choices, performing extensive sensitivity analyses and validation in economic evaluations. In resolving such gaps, the validity of future models can be increased to guide healthcare decision making in rare indications.
© 2024. The Author(s).
Conflict of interest statement
Angie Raad, Maria Rizzo and Katherine Appiah are full-time employees of Cytel. Isabella Kearns is a full-time employee of Takeda UK Ltd, and Luis Hernandez is a full-time employee of Takeda Pharmaceuticals America, Inc. This article reflects the views and opinions of the authors and not necessarily those of the organizations to which individuals are affiliated.
Figures




References
-
- American Cancer Society. What is lung cancer? 2023. https://www.cancer.org/cancer/lung-cancer/about/what-is.html.
-
- Surveillance Epidemiology and End Results Program. SEER cancer stat facts: lung and bronchus cancer. 2023. https://seer.cancer.gov/statfacts/html/lungb.html.
-
- National Cancer Institute. Non-Small Cell Lung Cancer Treatment (PDQ®)–Health Professional Version. 2023. https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

