Serum Troponin I Assessments in 5- to 30-Year-Olds After BNT162b2 Vaccination
- PMID: 38489117
- PMCID: PMC11058143
- DOI: 10.1007/s40121-024-00927-0
Serum Troponin I Assessments in 5- to 30-Year-Olds After BNT162b2 Vaccination
Abstract
Introduction: Rare myocarditis and pericarditis cases have occurred in coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine recipients. Troponin levels, a potential marker of myocardial injury, were assessed in healthy participants before and after BNT162b2 vaccination.
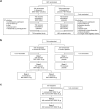
Methods: Vaccine-experienced 12- to 30-year-olds in phase 3 crossover C4591031 Substudy B (NCT04955626) who had two or three prior BNT162b2 30-μg doses were randomized to receive BNT162b2 30 μg followed by placebo, or placebo followed by BNT162b2 30 µg, 1 month apart. A participant subset, previously unvaccinated against COVID-19, in the phase 3 C4591007 study (NCT04816643) received up to three vaccinations (BNT162b2 10 μg or placebo [5- to 11-year-olds]) or open-label BNT162b2 30 μg (12- to 15-year-olds). Blood samples collected pre-vaccination, 4 days post-vaccination, and 1-month post-vaccination (C4591031 Substudy B only) were analyzed. Frequencies of elevated troponin I levels (male, > 35 ng/l; female, > 17 ng/l) were assessed.
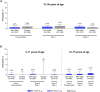
Results: Percentages of 12- to 30-year-olds (n = 1485) in C4591031 Substudy B with elevated troponin levels following BNT162b2 or placebo receipt were 0.5% and 0.8% before vaccination, 0.7% and 1.0% at day 4, and 0.7% and 0.5% at 1 month, respectively. In Study C4591007 (n = 1265), elevated troponin I levels were observed in 0.2, 0.4, and 0.2% of 5- to 11-year-old BNT162b2 recipients at baseline and 4 days post-dose 2 and 3, respectively; corresponding values in 12- to 15-year-olds were 0.4, 0.4, and 0.7%. No 5- to 11-year-old placebo recipients had elevated troponin levels. No myocarditis or pericarditis cases or deaths were reported.
Conclusions: Among 5- to < 30-year-olds in both studies, troponin levels were rarely elevated (≤ 1.0%) and similar before and post-vaccination; troponin levels were also similar between BNT162b2 and placebo in 12- to 30-year-old and 5- to 11-year-old recipients in the respective studies. No myocarditis or pericarditis cases were reported. These findings did not provide evidence that BNT162b2 causes troponin elevations. No utility of routine measurement of troponin levels in asymptomatic BNT162b2 recipients was identified.
Keywords: BNT162b2; COVID-19; Clinical trials; Myocarditis; Pericarditis; Safety; Troponin I; Vaccines.
© 2024. Pfizer Inc.
Conflict of interest statement
Timothy E. Albertson: None. J. Abraham Simón-Campos: AstraZeneca/Other-personal fees; Pfizer/Other-personal fees; Roche/Other-personal fees. Michael E. Dever: None. Jose F. Cardona: None. Essack Mitha: None. Jeffrey B. Baker: Pfizer/BioNTech/Other-received payment as a principal investigator for Study C4591007. Federico J. Mensa: BioNTech/Other-employee/Stock. Özlem Türeci: BioNTech/Board membership and board member/Advisor/Consultant/Honoraria/Intellectual property/Patents/Other-Cofounder and Chief Medical Officer/Stock. Uğur Şahin: BioNTech/Other-employee and board member/Stock. Caitlin Hansen, Smiti Bihari, Juleen Gayed, Xia Xu, Georgina Keep, Islamiat Oladipupo, Ye Feng, Hua Ma, Kenneth Koury, Susan Mather, Claudia Ana Ianos, Annaliesa S. Anderson, William C. Gruber, Charu Sabharwal, and Nicholas Kitchin are current or former employees of Pfizer and may hold stock or stock options.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

