Alzheimer's Disease and Cognitive Decline in Patients with Cardiovascular Diseases Along the Heart-Brain Axis
- PMID: 38489178
- PMCID: PMC11091595
- DOI: 10.3233/JAD-231096
Alzheimer's Disease and Cognitive Decline in Patients with Cardiovascular Diseases Along the Heart-Brain Axis
Abstract
Background: We hypothesize that Alzheimer's disease (AD)-related pathology may accelerate cognitive decline in patients with cardiovascular diseases.
Objective: To investigate the association between blood-based biomarkers of AD, astrocyte activation, and neurodegeneration and cognitive decline.
Methods: From the multi-center Heart-Brain study, we included 412 patients with heart failure, carotid occlusive disease or vascular cognitive impairment (age:68.6±9.0) and 128 reference participants (65.7±7.5). Baseline amyloid-β42/40 (Aβ42/40), phosphorylated-tau181 (pTau181), glial fibrillary acidic protein (GFAP), and neurofilament light (NfL) were determined using SiMoA (Quanterix). Memory, attention, language, and executive functioning were evaluated (follow-up:2.1±0.3 years). We applied linear mixed models with terms for biomarker, time and biomarker*time interactions, adjusted for age, sex, education, and site, to assess associations between biomarkers and cognitive decline.
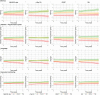
Results: Among patients, Aβ42/40 was not associated with cognitive performance at baseline. However, lower Aβ42/40 was associated with steeper decline in global cognition (β±SE:0.04±0.02). Higher pTau181 was associated with worse baseline performance on global cognition (-0.14±0.04) and memory (-0.31±0.09) and with steeper decline in global cognition (-0.07±0.02), memory (-0.09±0.04), attention (-0.05±0.02), and language (-0.10±0.03). Higher GFAP was associated with worse baseline performance on global cognition (-0.22±0.05), memory (-0.43±0.10), attention (-0.14±0.06), language (-0.15±0.05), and executive functioning (-0.15±0.05) and steeper decline in global cognition (-0.05±0.01). Higher NfL was associated with worse baseline performance on global cognition (-0.16±0.04), memory (-0.28±0.09), attention (-0.20±0.06), and executive functioning (-0.10±0.04), but was not associated with performance over time. In reference participants, no associations were found.
Conclusions: Our findings suggest that blood-based biomarkers of AD-related pathology predict cognitive decline in patients with cardiovascular diseases.
Keywords: Alzheimer’s disease; carotid stenosis; cognitive dysfunction; heart failure; vascular dementia.
Conflict of interest statement
Lieza G. Exalto is also employed at Julius Clinical.
Charlotte E. Teunissen is employed by Amsterdam UMC. She has grants or contracts for Research of the European Commission (Marie Curie International Training Network, grant agreement No 860197 (MIRIADE), Innovative Medicines Initiatives 3TR (Horizon 2020, grant no 831434) EPND (IMI 2 Joint Undertaking (JU), grant No. 101034344) and JPND (bPRIDE), National MS Society (Progressive MS alliance), Alzheimer Drug Discovery Foundation, Alzheimer Association, Health Holland, the Dutch Research Council (ZonMW), including TAP-dementia, a ZonMw funded project (#10510032120003) in the context of the Dutch National Dementia Strategy, Alzheimer Drug Discovery Foundation, The Selfridges Group Foundation, Alzheimer Netherlands. She is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). She is also a contract researcher for ADx Neurosciences, AC-Immune, Aribio, Axon Neurosciences, Beckman-Coulter, BioConnect, Bioorchestra, Brainstorm Therapeutics, Celgene, Cognition Therapeutics, EIP Pharma, Eisai, Eli Lilly Fujirebio, Grifols, Instant Nano Biosensors, Merck, Novo Nordisk, Olink, PeopleBio, Quanterix, Roche, Siemens, Toyama, Vivoryon, and the European Commission. She has received payment or honoraria from Roche, Novo Nordisk, and Grifols, where all payments were made to her institution. She also serves on editorial boards of Medidact Neurologie/Springer; and in Neurology: Neuroimmunology & Neuroinflammation. She is editor of Alzheimer Research and Therapy.
Research programs of Wiesje M. van Flier have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis-NL, Life-MI, AVID, Roche BV, Fujifilm, Eisai, Combinostics. She holds the Pasman chair. She is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). All funding is paid to her institution. She has been an invited speaker at Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council. All funding is paid to her institution. She is consultant to Oxford Health Policy Forum CIC, Roche, Biogen MA Inc, and Eisai. She is member of steering cie of NovoNordisk evoke/evoke+. All funding is paid to her institution. She participated in advisory boards of Biogen MA Inc, Roche, and Eli Lilly. She is member of the steering committee of EVOKE/EVOKE+(NovoNordisk). All funding is paid to her institution. She is member of the steering committee of PAVE, and Think Brain Health. She was associate editor of Alzheimer, Research & Therapy in 2020/2021. She is associate editor at Brain.
Calvin Trieu, Argonde C. van Harten, Anna E. Leeuwis, Astrid M. Hooghiemstra, Inge M. W. Verberk, Cor P. Allaart, Hans-Peter Brunner-La Rocca, L. Jaap Kappelle, Robert J. van Oostenbrugge, and Geert-Jan Biessels have no conflict of interest to report.
Figures

References
-
- Wolters FJ, Segufa RA, Darweesh SKL, Bos D, Ikram MA, Sabayan B, Hofman A, Sedaghat S (2018) Coronary heart disease, heart failure, and the risk of dementia: A systematic review and meta-analysis. Alzheimers Dement 14, 1493–1504. - PubMed
-
- Liu Y, Braidy N, Poljak A, Chan DKY, Sachdev P (2018) Cerebral small vessel disease and the risk of Alzheimer’s disease: A systematic review. Ageing Res Rev 47, 41–48. - PubMed
-
- Gottesman RF, Albert MS, Alonso A, Coker LH, Coresh J, Davis SM, Deal JA, McKhann GM, Mosley TH, Sharrett AR, Schneider ALC, Windham BG, Wruck LM, Knopman DS (2017) Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) Cohort. JAMA Neurol 74, 1246–1254. - PMC - PubMed
-
- Hooghiemstra AM, Bertens AS, Leeuwis AE, Bron EE, Bots ML, Brunner-La Rocca HP, de Craen AJM, van der Geest RJ, Greving JP, Kappelle LJ, Niessen WJ, van Oostenbrugge RJ, van Osch MJP, de Roos A, van Rossum AC, Biessels GJ, van Buchem MA, Daemen M, van der Flier WM (2017) The missing link in the pathophysiology of vascular cognitive impairment: Design of the Heart-Brain Study. Cerebrovasc Dis Extra 7, 140–152. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

