Semaphorin 7A coordinates neutrophil response during pulmonary inflammation and sepsis
- PMID: 38489236
- PMCID: PMC11157222
- DOI: 10.1182/bloodadvances.2023011778
Semaphorin 7A coordinates neutrophil response during pulmonary inflammation and sepsis
Abstract
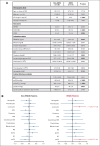
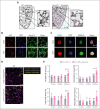
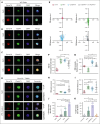
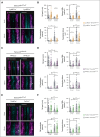
Pulmonary defense mechanisms are critical for host integrity during pneumonia and sepsis. This defense is fundamentally dependent on the activation of neutrophils during the innate immune response. Recent work has shown that semaphorin 7A (Sema7A) holds significant impact on platelet function, yet its role on neutrophil function within the lung is not well understood. This study aimed to identify the role of Sema7A during pulmonary inflammation and sepsis. In patients with acute respiratory distress syndrome (ARDS), we were able to show a correlation between Sema7A and oxygenation levels. During subsequent workup, we found that Sema7A binds to the neutrophil PlexinC1 receptor, increasing integrins, and L-selectin on neutrophils. Sema7A prompted neutrophil chemotaxis in vitro and the formation of platelet-neutrophil complexes in vivo. We also observed altered adhesion and transmigration of neutrophils in Sema7A-/-animals in the lung during pulmonary inflammation. This effect resulted in increased number of neutrophils in the interstitial space of Sema7A-/- animals but reduced numbers of neutrophils in the alveolar space during pulmonary sepsis. This finding was associated with significantly worse outcome of Sema7A-/- animals in a model of pulmonary sepsis. Sema7A has an immunomodulatory effect in the lung, affecting pulmonary sepsis and ARDS. This effect influences the response of neutrophils to external aggression and might influence patient outcome. This trial was registered at www.ClinicalTrials.gov as #NCT02692118.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures




References
-
- Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8(2):142–152. - PubMed

