Multifunctional Biocatalysts for Organic Synthesis
- PMID: 38489244
- PMCID: PMC10979396
- DOI: 10.1021/jacs.3c09542
Multifunctional Biocatalysts for Organic Synthesis
Abstract
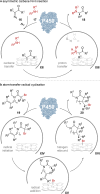
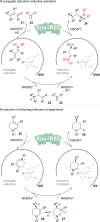
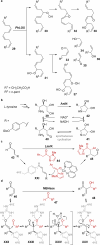
Biocatalysis is becoming an indispensable tool in organic synthesis due to high enzymatic catalytic efficiency as well as exquisite chemo- and stereoselectivity. Some biocatalysts display great promiscuity including a broad substrate scope as well as the ability to catalyze more than one type of transformation. These promiscuous activities have been applied individually to efficiently access numerous valuable target molecules. However, systems in which enzymes possessing multiple different catalytic activities are applied in the synthesis are less well developed. Such multifunctional biocatalysts (MFBs) would simplify chemical synthesis by reducing the number of operational steps and enzyme count, as well as simplifying the sequence space that needs to be engineered to develop an efficient biocatalyst. In this Perspective, we highlight recently reported MFBs focusing on their synthetic utility and mechanism. We also offer insight into their origin as well as comment on potential strategies for their discovery and engineering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Leveson-Gower R. B.; Mayer C.; Roelfes G. The Importance of Catalytic Promiscuity for Enzyme Design and Evolution. Nat. Rev. Chem. 2019, 3 (12), 687–705. 10.1038/s41570-019-0143-x. - DOI
-
- Camp J. E. Auto-Tandem Catalysis: Activation of Multiple, Mechanistically Distinct Process by a Single Catalyst. Eur. J. Org. Chem. 2017, 2017 (3), 425–433. 10.1002/ejoc.201600803. - DOI
-
- McIntosh J. A.; Liu Z.; Andresen B. M.; Marzijarani N. S.; Moore J. C.; Marshall N. M.; Borra-Garske M.; Obligacion J. V.; Fier P. S.; Peng F.; Forstater J. H.; Winston M. S.; An C.; Chang W.; Lim J.; Huffman M. A.; Miller S. P.; Tsay F.-R.; Altman M. D.; Lesburg C. A.; Steinhuebel D.; Trotter B. W.; Cumming J. N.; Northrup A.; Bu X.; Mann B. F.; Biba M.; Hiraga K.; Murphy G. S.; Kolev J. N.; Makarewicz A.; Pan W.; Farasat I.; Bade R. S.; Stone K.; Duan D.; Alvizo O.; Adpressa D.; Guetschow E.; Hoyt E.; Regalado E. L.; Castro S.; Rivera N.; Smith J. P.; Wang F.; Crespo A.; Verma D.; Axnanda S.; Dance Z. E. X.; Devine P. N.; Tschaen D.; Canada K. A.; Bulger P. G.; Sherry B. D.; Truppo M. D.; Ruck R. T.; Campeau L.-C.; Bennett D. J.; Humphrey G. R.; Campos K. R.; Maddess M. L. A Kinase-CGAS Cascade to Synthesize a Therapeutic STING Activator. Nature 2022, 603 (7901), 439–444. 10.1038/s41586-022-04422-9. - DOI - PubMed
-
- Huffman M. A.; Fryszkowska A.; Alvizo O.; Borra-Garske M.; Campos K. R.; Canada K. A.; Devine P. N.; Duan D.; Forstater J. H.; Grosser S. T.; Halsey H. M.; Hughes G. J.; Jo J.; Joyce L. A.; Kolev J. N.; Liang J.; Maloney K. M.; Mann B. F.; Marshall N. M.; McLaughlin M.; Moore J. C.; Murphy G. S.; Nawrat C. C.; Nazor J.; Novick S.; Patel N. R.; Rodriguez-Granillo A.; Robaire S. A.; Sherer E. C.; Truppo M. D.; Whittaker A. M.; Verma D.; Xiao L.; Xu Y.; Yang H. Design of an in Vitro Biocatalytic Cascade for the Manufacture of Islatravir. Science (1979) 2019, 366 (6470), 1255.10.1126/science.aay8484. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

