Detection of pathogenic Leptospira with rapid extraction followed by recombinase polymerase amplification (RPA) and quantitative polymerase chain reaction (qPCR) assay-A comprehensive study from Sri Lanka
- PMID: 38489285
- PMCID: PMC10942058
- DOI: 10.1371/journal.pone.0295287
Detection of pathogenic Leptospira with rapid extraction followed by recombinase polymerase amplification (RPA) and quantitative polymerase chain reaction (qPCR) assay-A comprehensive study from Sri Lanka
Abstract
Leptospirosis is the most widespread zoonosis in the world. The disease is more prevalent in tropical regions where the majority of developing countries are located. Leptospirosis is considered a protean manifestation zoonosis with severity of the disease ranging from a mild febrile illness to a severe and life-threatening illness. Clinical symptoms of leptospirosis overlap with other tropical febrile illnesses. Early, rapid, and definitive diagnosis is important for effective patient management. Since Polymerase Chain Reaction (PCR)-based assays are not readily available in most clinical settings, there is a need for an affordable, simple, and rapid diagnostic test. Quantitative PCR (qPCR) and Recombinase Polymerase Amplification (RPA) were implemented at the Faculty of Medicine, University of Kelaniya, and a prospective study to evaluate RPA for diagnosis of acute phase of leptospirosis was conducted. Results indicate that RPA and qPCR were positive in 81% (98/121) of the total positive and acute clinical samples. Of the 81 positive MAT confirmed patients 60 (74%) and 53 (65%) were positive with qPCR and RPA respectively. Retrospective evaluation revealed a high diagnostic accuracy (sensitivity-70% and specificity-87%) of RPA compared to MAT as the reference gold standard. Results further suggest that there is no significant difference between the two assays, qPCR and RPA-SwiftX (P = 0.40). Laboratory procedures for the extraction and detection by qPCR in the laboratory have been optimized to obtain results within 6 hours. However, the RPA-SwiftX method under field conditions took 35 minutes. The RPA-SwiftX method could replace the qPCR which shows similar sensitivity and specificity. Therefore, RPA established under the current study presents a powerful tool for the early and rapid diagnosis of leptospirosis at point-of-care.
Copyright: © 2024 Uduwawala et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
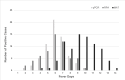
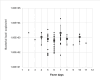
References
-
- World Health Organization. Report of the first meeting of the Leptospirosis Burden Epidemiology Reference Group. 2010.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

