Gut bacteria-derived serotonin promotes immune tolerance in early life
- PMID: 38489352
- PMCID: PMC11328322
- DOI: 10.1126/sciimmunol.adj4775
Gut bacteria-derived serotonin promotes immune tolerance in early life
Abstract
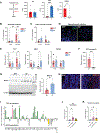
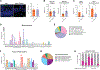
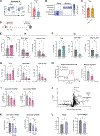
The gut microbiota promotes immune system development in early life, but the interactions between the gut metabolome and immune cells in the neonatal gut remain largely undefined. Here, we demonstrate that the neonatal gut is uniquely enriched with neurotransmitters, including serotonin, and that specific gut bacteria directly produce serotonin while down-regulating monoamine oxidase A to limit serotonin breakdown. We found that serotonin directly signals to T cells to increase intracellular indole-3-acetaldehdye and inhibit mTOR activation, thereby promoting the differentiation of regulatory T cells, both ex vivo and in vivo in the neonatal intestine. Oral gavage of serotonin into neonatal mice resulted in long-term T cell-mediated antigen-specific immune tolerance toward both dietary antigens and commensal bacteria. Together, our study has uncovered an important role for specific gut bacteria to increase serotonin availability in the neonatal gut and identified a function of gut serotonin in shaping T cell response to dietary antigens and commensal bacteria to promote immune tolerance in early life.
Conflict of interest statement
Figures


References
-
- Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M, The First Microbial colonizers of the human gut: Composition, Activities, and Health Implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 81, (2017). - PMC - PubMed
-
- Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao LJ,Zhang YS, Kotowska D, Colding C, Tremaroli V, Yi Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J, Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703 (2015). - PubMed
-
- Sharon G, Cruz NJ, Kang DW, Gandal MJ, Wang B, Kim YM, Zink EM, Casey CP, Taylor BC, Lane CJ, Bramer LM, Isern NG, Hoyt DW, Noecker C, Sweredoski MJ, Moradian A, Borenstein E, Jansson JK, Knight R, Metz TO, Lois C, Geschwind DH, Krajmalnik-Brown R, Mazmanian SK, Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cell 177, 1600–1618.e17 (2019). - PMC - PubMed
-
- Levan SR, Stamnes KA, Lin DL, Panzer AR, Fukui E, McCauley K, Fujimura KE, McKean M, Ownby DR, Zoratti EM, Boushey HA, Cabana MD, Johnson CC, Lynch SV, Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 4, 1851–1861 (2019). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK135816/DK/NIDDK NIH HHS/United States
- T32 DK116970/DK/NIDDK NIH HHS/United States
- TL1 TR002386/TR/NCATS NIH HHS/United States
- K01 DK114376/DK/NIDDK NIH HHS/United States
- DP2 HD101401/HD/NICHD NIH HHS/United States
- R01 HL169989/HL/NHLBI NIH HHS/United States
- R01 DK132244/DK/NIDDK NIH HHS/United States
- F32 HD112151/HD/NICHD NIH HHS/United States
- R01 AI172027/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- KL2 TR002385/TR/NCATS NIH HHS/United States
- R01 HD110118/HD/NICHD NIH HHS/United States
- R21 CA270998/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous