The temporal and genomic scale of selection following hybridization
- PMID: 38489387
- PMCID: PMC10962946
- DOI: 10.1073/pnas.2309168121
The temporal and genomic scale of selection following hybridization
Abstract
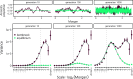
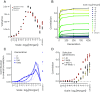
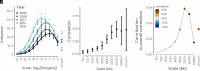
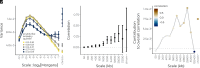
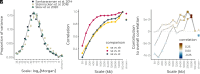
Genomic evidence supports an important role for selection in shaping patterns of introgression along the genome, but frameworks for understanding the evolutionary dynamics within hybrid populations that underlie these patterns have been lacking. Due to the clock-like effect of recombination in hybrids breaking up parental haplotypes, drift and selection produce predictable patterns of ancestry variation at varying spatial genomic scales through time. Here, we develop methods based on the Discrete Wavelet Transform to study the genomic scale of local ancestry variation and its association with recombination rates and show that these methods capture temporal dynamics of drift and genome-wide selection after hybridization. We apply these methods to published datasets from hybrid populations of swordtail fish (Xiphophorus) and baboons (Papio) and to inferred Neanderthal introgression in modern humans. Across systems, upward of 20% of variation in local ancestry at the broadest genomic scales can be attributed to systematic selection against introgressed alleles, consistent with strong selection acting on early-generation hybrids. Signatures of selection at fine genomic scales suggest selection over longer time scales; however, we suggest that our ability to confidently infer selection at fine scales is likely limited by inherent biases in current methods for estimating local ancestry from contiguous segments of genomic similarity. Wavelet approaches will become widely applicable as genomic data from systems with introgression become increasingly available and can help shed light on generalities of the genomic consequences of interspecific hybridization.
Keywords: Neanderthal; hybridization; introgression; wavelet transform.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
The temporal and genomic scale of selection following hybridization.bioRxiv [Preprint]. 2023 May 26:2023.05.25.542345. doi: 10.1101/2023.05.25.542345. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Mar 19;121(12):e2309168121. doi: 10.1073/pnas.2309168121. PMID: 37337589 Free PMC article. Updated. Preprint.
References
-
- Harrison R. G., Larson E. L., Hybridization, introgression, and the nature of species boundaries. J. Heredity 105, 795–809 (2014). - PubMed
-
- Barton N., Bengtsson B. O., The barrier to genetic exchange between hybridising populations. Heredity 57, 357–376 (1986). - PubMed
-
- Wu C. I., The genic view of the process of speciation. J. Evol. Biol. 14, 851–865 (2001).
-
- Ravinet M., et al. , Interpreting the genomic landscape of speciation: A road map for finding barriers to gene flow. J. Evol. Biol. 30, 1450–1477 (2017). - PubMed

