ATF3-CBS signaling axis coordinates ferroptosis and tumorigenesis in colorectal cancer
- PMID: 38490069
- PMCID: PMC10958616
- DOI: 10.1016/j.redox.2024.103118
ATF3-CBS signaling axis coordinates ferroptosis and tumorigenesis in colorectal cancer
Abstract
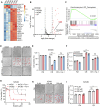
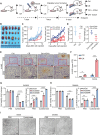
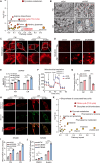
The induction of ferroptosis is promising for cancer therapy. However, the mechanisms enabling cancer cells to evade ferroptosis, particularly in low-cystine environments, remain elusive. Our study delves into the intricate regulatory mechanisms of Activating transcription factor 3 (ATF3) on Cystathionine β-synthase (CBS) under cystine deprivation stress, conferring resistance to ferroptosis in colorectal cancer (CRC) cells. Additionally, our findings establish a positively correlation between this signaling axis and CRC progression, suggesting its potential as a therapeutic target. Mechanistically, ATF3 positively regulates CBS to resist ferroptosis under cystine deprivation stress. In contrast, the suppression of CBS sensitizes CRC cells to ferroptosis through targeting the mitochondrial tricarboxylic acid (TCA) cycle. Notably, our study highlights that the ATF3-CBS signaling axis enhances ferroptosis-based CRC cancer therapy. Collectively, the findings reveal that the ATF3-CBS signaling axis is the primary feedback pathway in ferroptosis, and blocking this axis could be a potential therapeutic approach for colorectal cancer.
Keywords: ATF3-CBS signaling axis; Colorectal cancer (CRC); Ferroptosis; Mitochondrial tricarboxylic acid (TCA) cycle.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
METTL17 coordinates ferroptosis and tumorigenesis by regulating mitochondrial translation in colorectal cancer.Redox Biol. 2024 May;71:103087. doi: 10.1016/j.redox.2024.103087. Epub 2024 Feb 13. Redox Biol. 2024. PMID: 38377789 Free PMC article.
-
Induction of ferroptosis by ATF3 elevation alleviates cisplatin resistance in gastric cancer by restraining Nrf2/Keap1/xCT signaling.Cell Mol Biol Lett. 2021 Jun 7;26(1):26. doi: 10.1186/s11658-021-00271-y. Cell Mol Biol Lett. 2021. PMID: 34098867 Free PMC article.
-
Activation of the reverse transsulfuration pathway through NRF2/CBS confers erastin-induced ferroptosis resistance.Br J Cancer. 2020 Jan;122(2):279-292. doi: 10.1038/s41416-019-0660-x. Epub 2019 Dec 10. Br J Cancer. 2020. PMID: 31819185 Free PMC article.
-
The Dual Roles of Activating Transcription Factor 3 (ATF3) in Inflammation, Apoptosis, Ferroptosis, and Pathogen Infection Responses.Int J Mol Sci. 2024 Jan 9;25(2):824. doi: 10.3390/ijms25020824. Int J Mol Sci. 2024. PMID: 38255898 Free PMC article. Review.
-
Ferroptosis in colorectal cancer: a future target?Br J Cancer. 2023 Apr;128(8):1439-1451. doi: 10.1038/s41416-023-02149-6. Epub 2023 Jan 26. Br J Cancer. 2023. PMID: 36703079 Free PMC article. Review.
Cited by
-
Inhibition of p16 and NF-κB Oncogenic Activity in Human Papillomavirus-Infected Cervical Cancer Cells: A New Role for Activating Transcription Factor-3.Yale J Biol Med. 2025 Mar 31;98(1):21-32. doi: 10.59249/XCAB1680. eCollection 2025 Mar. Yale J Biol Med. 2025. PMID: 40165810 Free PMC article.
-
CircWDR78 inhibits the development of colorectal cancer by regulating the miR-653-3p/RGS4 axis.J Cancer Res Clin Oncol. 2025 Feb 6;151(2):66. doi: 10.1007/s00432-025-06092-2. J Cancer Res Clin Oncol. 2025. PMID: 39912944 Free PMC article.
-
Influence of Mesalazine on Ferroptosis-Related Gene Expression in In Vitro Colorectal Cancer Culture.Biomedicines. 2025 Jan 16;13(1):219. doi: 10.3390/biomedicines13010219. Biomedicines. 2025. PMID: 39857803 Free PMC article.
-
Glucose Metabolic Reprogramming in Colorectal Cancer: From Mechanisms to Targeted Therapy Approaches.Cancer Med. 2025 Sep;14(17):e71185. doi: 10.1002/cam4.71185. Cancer Med. 2025. PMID: 40884151 Free PMC article. Review.
-
The role of SIRT1 in autophagy and drug resistance: unveiling new targets and potential biomarkers in cancer therapy.Front Pharmacol. 2024 Sep 30;15:1469830. doi: 10.3389/fphar.2024.1469830. eCollection 2024. Front Pharmacol. 2024. PMID: 39403142 Free PMC article. Review.
References
-
- Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100(1):57–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

