TGF-β-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer
- PMID: 38490994
- PMCID: PMC10943116
- DOI: 10.1038/s41419-024-06594-w
TGF-β-driven LIF expression influences neutrophil extracellular traps (NETs) and contributes to peritoneal metastasis in gastric cancer
Abstract
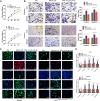
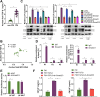
Gastric cancer (GC), notorious for its poor prognosis, often advances to peritoneal dissemination, a crucial determinant of detrimental outcomes. This study intricately explores the role of the TGFβ-Smad-LIF axis within the tumor microenvironment in propagating peritoneal metastasis, with a specific emphasis on its molecular mechanism in instigating Neutrophil Extracellular Traps (NETs) formation and encouraging GC cellular functions. Through a blend of bioinformatics analyses, utilizing TCGA and GEO databases, and meticulous in vivo and in vitro experiments, LIF was identified as pivotally associated with GC metastasis, notably, enhancing the NETs formation through neutrophil stimulation. Mechanistically, TGF-β was substantiated to elevate LIF expression via the activation of the Smad2/3 complex, culminating in NETs formation and consequently, propelling peritoneal metastasis of GC. This revelation uncovers a novel potential therapeutic target, promising a new avenue in managing GC and mitigating its metastatic propensities.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

