Bearded capuchin monkeys as a model for Alzheimer's disease
- PMID: 38491154
- PMCID: PMC10943096
- DOI: 10.1038/s41598-024-56791-y
Bearded capuchin monkeys as a model for Alzheimer's disease
Abstract
The absence of a natural animal model is one of the main challenges in Alzheimer's disease research. Despite the challenges of using nonhuman primates in studies, these animals can bridge mouse models and humans, as nonhuman primates are phylogenetically closer to humans and can spontaneously develop AD-type pathology. The capuchin monkey, a New World primate, has recently attracted attention due to its skill in creating and using instruments. We analyzed one capuchin brain using structural 7 T MRI and performed a neuropathological evaluation of three animals. Alzheimer-type pathology was found in the two of the capuchins. Widespread β-amyloid pathology was observed, mainly in focal deposits with variable morphology and a high density of mature plaques. Notably, plaque-associated dystrophic neurites associated with disruption of axonal transport and early cytoskeletal alteration were frequently found. Unlike in other species of New World monkeys, cerebral arterial angiopathy was not the predominant form of β-amyloid pathology. Additionally, abnormal aggregates of hyperphosphorylated tau, resembling neurofibrillary pathology, were observed in the temporal and frontal cortex. Astrocyte hypertrophy surrounding plaques was found, suggesting a neuroinflammatory response. These findings indicate that aged capuchin monkeys can spontaneously develop Alzheimer-type pathology, indicating that they may be an advantageous animal model for research in Alzheimer's disease.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
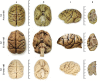

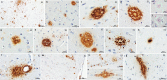


Update of
-
Bearded capuchin monkey as a model for Alzheimer's disease research.Res Sq [Preprint]. 2023 Dec 6:rs.3.rs-3495799. doi: 10.21203/rs.3.rs-3495799/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 15;14(1):6287. doi: 10.1038/s41598-024-56791-y. PMID: 38106066 Free PMC article. Updated. Preprint.
Similar articles
-
Bearded capuchin monkey as a model for Alzheimer's disease research.Res Sq [Preprint]. 2023 Dec 6:rs.3.rs-3495799. doi: 10.21203/rs.3.rs-3495799/v1. Res Sq. 2023. Update in: Sci Rep. 2024 Mar 15;14(1):6287. doi: 10.1038/s41598-024-56791-y. PMID: 38106066 Free PMC article. Updated. Preprint.
-
Relationship between apolipoprotein E and the amyloid deposits and dystrophic neurites of Alzheimer's disease.Neuropathol Appl Neurobiol. 1997 Dec;23(6):483-91. doi: 10.1111/j.1365-2990.1997.tb01325.x. Neuropathol Appl Neurobiol. 1997. PMID: 9460714
-
Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology.Brain. 2010 May;133(Pt 5):1312-27. doi: 10.1093/brain/awq056. Epub 2010 Mar 31. Brain. 2010. PMID: 20360050 Free PMC article. Clinical Trial.
-
Brain aging and Alzheimer's disease, a perspective from non-human primates.Aging (Albany NY). 2024 Oct 29;16(20):13145-13171. doi: 10.18632/aging.206143. Epub 2024 Oct 29. Aging (Albany NY). 2024. PMID: 39475348 Free PMC article. Review.
-
[Transmission of pathogenic protein aggregates in Alzheimer's disease].Mol Biol (Mosk). 2017 May-Jun;51(3):418-422. doi: 10.7868/S0026898417030144. Mol Biol (Mosk). 2017. PMID: 28707657 Review. Russian.
Cited by
-
The neuropathologic basis for translational biomarker development in the macaque model of late-onset Alzheimer's disease.J Alzheimers Dis. 2025 Apr;104(4):1243-1258. doi: 10.1177/13872877251323787. Epub 2025 Mar 17. J Alzheimers Dis. 2025. PMID: 40095666 Free PMC article.

