Drug stewardship in chronic kidney disease to achieve effective and safe medication use
- PMID: 38491222
- PMCID: PMC11929520
- DOI: 10.1038/s41581-024-00823-3
Drug stewardship in chronic kidney disease to achieve effective and safe medication use
Abstract
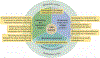
People living with chronic kidney disease (CKD) often experience multimorbidity and require polypharmacy. Kidney dysfunction can also alter the pharmacokinetics and pharmacodynamics of medications, which can modify their risks and benefits; the extent of these changes is not well understood for all situations or medications. The principle of drug stewardship is aimed at maximizing medication safety and effectiveness in a population of patients through a variety of processes including medication reconciliation, medication selection, dose adjustment, monitoring for effectiveness and safety, and discontinuation (deprescribing) when no longer necessary. This Review is aimed at serving as a resource for achieving optimal drug stewardship for patients with CKD. We describe special considerations for medication use during pregnancy and lactation, during acute illness and in patients with cancer, as well as guidance for the responsible use of over-the-counter drugs, herbal remedies, supplements and sick-day rules. We also highlight inequities in medication access worldwide and suggest policies to improve access to quality and essential medications for all persons with CKD. Further strategies to promote drug stewardship include patient education and engagement, the use of digital health tools, shared decision-making and collaboration within interdisciplinary teams. Throughout, we position the person with CKD at the centre of all drug stewardship efforts.
© 2024. Springer Nature Limited.
Conflict of interest statement
• RK reports receiving honoraria from Baxter.
• SBA reports funding from the Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada. She is an advisory board member of the Canadian Institutes of Health Research Institute of Gender and Health and the Governance Council for the Canadian Medical Association Journal. She is the President-Elect for the Organization for the Study of Sex Differences.
• LAI reports funding from NIH, National Kidney Foundation (NKF), Omeros, Chinnocks. and Reata Pharmaceuticals for research and contracts to Tufts Medical Center; and consulting agreements to Diamerix.
• EFB reports funding from NIH, the Agency for Healthcare Research and Quality, and Numares. She consults for Wolters Kluwer.
• CMC has received consultation, advisory board membership, honoraria, or research funding from Sanofi, Pfizer, Leo Pharma, Astellas, Janssen, Amgen, Boehringer-Ingelheim, Baxter and, through LiV Academy, AstraZeneca. She is editor in chief of the Canadian Journal of Kidney Health and Disease (CJKH).
• JJC has conducted pharmacoepidemiological studies for which Karolinska Institutet has received support from AstraZeneca, ViforPharma, Novonordisk, Astellas, MSD, GSK, Boehringer Ingelheim and Amgen; He also reports receiving lecture fees from Baxter, Fresenius Kabi, AstraZeneca, Astellas, GSK and Abbott; and participation in advisory boards for Astrazeneca, Nestle and Bayer. He is funded by the Swedish Research Council, the National Institutes of Health (NIH), and Swedish Heart and Lung Foundation.
• The rest of authors do not have any conflict of interest to report.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

