Persistent COVID-19 parosmia and olfactory loss post olfactory training: randomized clinical trial comparing central and peripheral-acting therapeutics
- PMID: 38492007
- PMCID: PMC11211159
- DOI: 10.1007/s00405-024-08548-6
Persistent COVID-19 parosmia and olfactory loss post olfactory training: randomized clinical trial comparing central and peripheral-acting therapeutics
Abstract
Purpose: Although COVID-19 anosmia is often transient, patients with persistent olfactory dysfunction (pOD) can experience refractory parosmia and diminished smell. This study evaluated four putative therapies for parosmia in patients with chronic COVID-19 olfactory impairment.
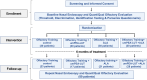
Methods: After screening nasal endoscopy, 85 patients (49 female, 58%) with pOD and treatment-refractory parosmia were randomized to: (1) ultramicronized palmitoylethanolamide and luteolin + olfactory training (OT) (umPEALUT group, n = 17), (2) alpha-lipoic acid + OT (ALA group, n = 21), (3) umPEALUT + ALA + OT (combination group, n = 28), or 4) olfactory training (OT) alone (control group, n = 23). Olfactory function was assessed at baseline (T0) and 6 months (T1) using a parosmia questionnaire and Sniffin' Sticks test of odor threshold, detection, and identification (TDI). Analyses included one-way ANOVA for numeric data and Chi-Square analyses for nominal data on parosmia.
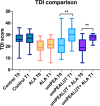
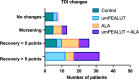
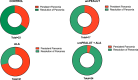
Results: The umPEALUT group had the largest improvement in TDI scores (21.8 ± 9.4 to 29.7 ± 7.5) followed by the combination group (19.6 ± 6.29 to 27.5 ± 2.7), both p < 0.01. The control and ALA groups had no significant change. Patients in the combination and umPEALUT groups had significantly improved TDI scores compared to ALA and control groups (p < 0.001). Rates of parosmia resolution after 6 months were reported at 96% for combination, 65% for control, 53% for umPEALUT and 29% for ALA (p < 0.001). All treatment regimens were well-tolerated.
Conclusions: umPEALUT and OT, with or without ALA, was associated with improvement in TDI scores and parosmia, whereas OT alone or OT with ALA were associated with little benefit.
Keywords: Alpha lipoic acid; Anosmia; Coronavirus long-COVID; Palmitoylethanolamide; Parosmia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Treatment of COVID-19 olfactory dysfunction with olfactory training, palmitoylethanolamide with luteolin, or combined therapy: a blinded controlled multicenter randomized trial.Eur Arch Otorhinolaryngol. 2023 Nov;280(11):4949-4961. doi: 10.1007/s00405-023-08085-8. Epub 2023 Jun 28. Eur Arch Otorhinolaryngol. 2023. PMID: 37380908 Free PMC article. Clinical Trial.
-
Platelet-Rich Plasma for Treating COVID-19-Related Anosmia, Hyposmia, and Parosmia: A Controlled Longitudinal Study.Otolaryngol Head Neck Surg. 2025 Apr;172(4):1450-1458. doi: 10.1002/ohn.1149. Epub 2025 Jan 31. Otolaryngol Head Neck Surg. 2025. PMID: 39888025 Clinical Trial.
-
Ultramicronized Palmitoylethanolamide and Luteolin Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial.Curr Neuropharmacol. 2022;20(10):2001-2012. doi: 10.2174/1570159X20666220420113513. Curr Neuropharmacol. 2022. PMID: 35450527 Free PMC article. Clinical Trial.
-
Parosmia: Pathophysiology and Management.Curr Allergy Asthma Rep. 2025 Jan 16;25(1):10. doi: 10.1007/s11882-024-01189-z. Curr Allergy Asthma Rep. 2025. PMID: 39821581 Review.
-
Therapeutic options of post-COVID-19 related olfactory dysfunction: a systematic review and meta-analysis.Rhinology. 2023 Feb 1;61(1):2-11. doi: 10.4193/Rhin22.221. Rhinology. 2023. PMID: 36173148
Cited by
-
Effects of therapeutic interventions on long COVID: a meta-analysis of randomized controlled trials.EClinicalMedicine. 2025 Aug 5;87:103412. doi: 10.1016/j.eclinm.2025.103412. eCollection 2025 Sep. EClinicalMedicine. 2025. PMID: 40808967 Free PMC article.
-
Stellate Ganglion Block for the Treatment of COVID-19-Induced Parosmia: A Randomized Clinical Trial.JAMA Otolaryngol Head Neck Surg. 2025 Aug 1;151(8):741-749. doi: 10.1001/jamaoto.2025.1304. JAMA Otolaryngol Head Neck Surg. 2025. PMID: 40504522
-
Therapeutic options for the treatment of post-acute sequelae of COVID-19: a scoping review.BMC Infect Dis. 2025 May 22;25(1):731. doi: 10.1186/s12879-025-11131-x. BMC Infect Dis. 2025. PMID: 40405092 Free PMC article.
-
Palmitoylethanolamide supplementation for human health: A state-of-the-art systematic review of Randomized Controlled Trials in patient populations.Brain Behav Immun Health. 2024 Dec 23;43:100927. doi: 10.1016/j.bbih.2024.100927. eCollection 2025 Feb. Brain Behav Immun Health. 2024. PMID: 39839988 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

