Use of tryptic peptide MALDI mass spectrometry imaging to identify the spatial proteomic landscape of colorectal cancer liver metastases
- PMID: 38492056
- PMCID: PMC10944452
- DOI: 10.1007/s10238-024-01311-5
Use of tryptic peptide MALDI mass spectrometry imaging to identify the spatial proteomic landscape of colorectal cancer liver metastases
Abstract
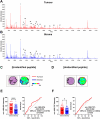
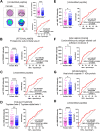
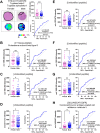
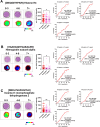
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths worldwide. CRC liver metastases (CRLM) are often resistant to conventional treatments, with high rates of recurrence. Therefore, it is crucial to identify biomarkers for CRLM patients that predict cancer progression. This study utilised matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) in combination with liquid chromatography-tandem mass spectrometry (LC-MS/MS) to spatially map the CRLM tumour proteome. CRLM tissue microarrays (TMAs) of 84 patients were analysed using tryptic peptide MALDI-MSI to spatially monitor peptide abundances across CRLM tissues. Abundance of peptides was compared between tumour vs stroma, male vs female and across three groups of patients based on overall survival (0-3 years, 4-6 years, and 7+ years). Peptides were then characterised and matched using LC-MS/MS. A total of 471 potential peptides were identified by MALDI-MSI. Our results show that two unidentified m/z values (1589.876 and 1092.727) had significantly higher intensities in tumours compared to stroma. Ten m/z values were identified to have correlation with biological sex. Survival analysis identified three peptides (Histone H4, Haemoglobin subunit alpha, and Inosine-5'-monophosphate dehydrogenase 2) and two unidentified m/z values (1305.840 and 1661.060) that were significantly higher in patients with shorter survival (0-3 years relative to 4-6 years and 7+ years). This is the first study using MALDI-MSI, combined with LC-MS/MS, on a large cohort of CRLM patients to identify the spatial proteome in this malignancy. Further, we identify several protein candidates that may be suitable for drug targeting or for future prognostic biomarker development.
Keywords: Biomarkers; Colorectal cancer; Colorectal cancer liver metastasis; Drug targets; LC-MS/MS; MALDI-MSI.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



References
-
- Morgan E, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–44. 10.1136/gutjnl-2022-327736. - PubMed
-
- Dong Y, Gruenberger T. Surgical management of colorectal liver metastases—a practical clinical approach. European Surgery. 2023;55(3):94–9. 10.1007/s10353-023-00796-w.
MeSH terms
Substances
Grants and funding
- Postgraduate Research Scholarship/University of Adelaide
- Infrastructure Grant/Cancer Council SA Beat Cancer
- Infrastructure Grant/Cancer Council SA Beat Cancer
- Matched Infrastructure Support Grant/The Hospital Research Foundation Group
- Matched Infrastructure Support Grant/The Hospital Research Foundation Group
LinkOut - more resources
Full Text Sources
Medical

