Protocol for quantifying pyramidal neuron hyperexcitability in a mouse model of neurodevelopmental encephalopathy
- PMID: 38492227
- PMCID: PMC10959716
- DOI: 10.1016/j.xpro.2024.102954
Protocol for quantifying pyramidal neuron hyperexcitability in a mouse model of neurodevelopmental encephalopathy
Abstract
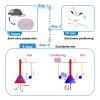
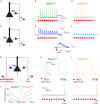
Here, we present a protocol for quantifying pyramidal neuron hyperexcitability in a mouse model of STXBP1 neurodevelopmental encephalopathy (Stxbp1hap). We describe steps for preparing brain slices, positioning electrodes, and performing an excitability test to investigate microcircuit failures. This protocol is based on recording layer 2/3 cortical pyramidal neurons in response to stimulation of two independent sets of excitatory axons that recruit feedforward inhibition microcircuits. For complete details on the use and execution of this protocol, please refer to Dos Santos et al.1.
Keywords: Genetics; Health Sciences; Neuroscience.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- Kovacevic J., Maroteaux G., Schut D., Loos M., Dubey M., Pitsch J., Remmelink E., Koopmans B., Crowley J., Cornelisse L.N., et al. Protein instability, haploinsufficiency, and cortical hyper-excitability underlie STXBP1 encephalopathy. Brain. 2018;141:1350–1374. doi: 10.1093/brain/awy046. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

