Targeting hematologic malignancies by inhibiting E-selectin: A sweet spot for AML therapy?
- PMID: 38493006
- PMCID: PMC11051645
- DOI: 10.1016/j.blre.2024.101184
Targeting hematologic malignancies by inhibiting E-selectin: A sweet spot for AML therapy?
Abstract
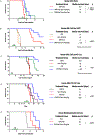
E-selectin, a cytoadhesive glycoprotein, is expressed on venular endothelial cells and mediates leukocyte localization to inflamed endothelium, the first step in inflammatory cell extravasation into tissue. Constitutive marrow endothelial E-selectin expression also supports bone marrow hematopoiesis via NF-κB-mediated signaling. Correspondingly, E-selectin interaction with E-selectin ligand (sialyl Lewisx) on acute myeloid leukemia (AML) cells leads to chemotherapy resistance in vivo. Uproleselan (GMI-1271) is a carbohydrate analog of sialyl Lewisx that blocks E-selectin binding. A Phase 2 trial of MEC chemotherapy combined with uproleselan for relapsed/refractory AML showed a median overall survival of 8.8 months and low (2%) rates of severe oral mucositis. Clinical trials seek to confirm activity in AML and mitigation of neutrophil-mediated adverse events (mucositis and diarrhea) after intensive chemotherapy. In this review we summarize E-selectin biology and the rationale for uproleselan in combination with other therapies for hematologic malignancies. We also describe uproleselan pharmacology and ongoing clinical trials.
Keywords: Acute myeloid leukemia (AML); E-selectin; Hematologic malignancies; Selectin; Uproleselan.
Copyright © 2024 GlycoMimetics, Inc. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Geoffrey L. Uy: Advisory Board, Jazz Pharmaceuticals. Daniel J. DeAngelo: Consultancy for Amgen, Autolos, Agios, Blueprint, FortySeven, Gilead, Incyte, Jazz Pharmaceuticals, Novartis, Pfizer, Servier and Takeda. Research funding from Abbvie, GlycoMimetics, Novartis, and Blueprint Pharmaceuticals. Data Safety Monitoring Boards for Daiichi-Sankyo, Fibrogen, Mt. Sinai Myeloproliferative Neoplasms Consortium. Jay N. Lozier: Employment and equity position in GlycoMimetics, Inc. Dennis M Fisher: Consultancy for GlycoMimetics, Inc. Brian A Jonas: Consultant/advisor for AbbVie, BMS, Daiichi Sankyo, Genentech, Gilead, GlycoMimetics, Kymera, Pfizer, Rigel, Servier, and Takeda; protocol steering committee for GlycoMimetics; data monitoring committee for Gilead; travel reimbursement/support from Rigel; institutional research funding from AbbVie, Amgen, Aptose, AROG, BMS, Celgene, Daiichi Sankyo, F. Hoffmann-La Roche, Forma, Forty-Seven, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune-Onc, Incyte, Jazz, Loxo Oncology, Pfizer, Pharmacyclics, Sigma Tau, and Treadwell. John L. Magnani: Employment, consultancy, and equity positions in GlycoMimetics, Inc., and Glycotech, Inc. Pamela S. Becker: Research funding from Glycomimetics, Notable Labs, Pfizer and GPCR. Medical Advisor to Accordant Health Services. Hillard M. Lazarus: Consultancy for Actinium Pharmaceuticals, CSL Behring, GlycoMimetics Inc., Jazz Pharmaceuticals, Partner Therapeutics, Pluristem Therapeutics Inc., and Seattle Genetics. Equity position in Partner Therapeutics. Speakers' bureau for AstraZeneca and Bristol-Myers Squibb. Data Safety Monitoring Board for BioSight and Bristol-Myers Squibb. Ingrid G. Winkler: Patents and royalties on GlycoMimetics, Inc. compounds. The authors thank Drew Provan for excellent graphic design and rendering of figures, Ed Rock and Kerry Woodford for careful review of the manuscript, Scott Brittain for help generating Supplemental Figs. 1 and 2, and Shanti Rodriguez for help in preparation of the manuscript.
Figures


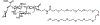

References
-
- U.S. Food & Drug Administration. FDA approval of cytarabine. Available from, https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview....
-
- Bailey C, et al. Cytosine arabinoside in the treatment of acute myeloblastic leukaemia. Lancet 1971;297(7712):1268–71. - PubMed
-
- Rudnick SA, et al. High dose cytosine arabinoside (HDARAC) in refractory acute leukemia. Cancer 1979;44(4):1189–93. - PubMed
-
- Rai KR, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood 1981;58(6):1203–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

