Long term results of a prospective multicenter observational study on the use of anti-human T-lymphocyte immunoglobulin (ATLG) in unrelated donor transplantation (ATOS study)
- PMID: 38493275
- PMCID: PMC11226393
- DOI: 10.1038/s41409-024-02264-9
Long term results of a prospective multicenter observational study on the use of anti-human T-lymphocyte immunoglobulin (ATLG) in unrelated donor transplantation (ATOS study)
Erratum in
-
Correction: Long term results of a prospective multicenter observational study on the use of anti-human T-lymphocyte immunoglobulin (ATLG) in unrelated donor transplantation (ATOS study).Bone Marrow Transplant. 2024 Aug;59(8):1204. doi: 10.1038/s41409-024-02274-7. Bone Marrow Transplant. 2024. PMID: 38654115 Free PMC article. No abstract available.
Abstract
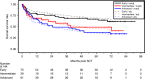
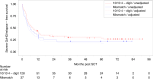
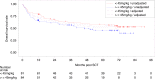
ATOS is a prospective observational study evaluating the outcome of patients receiving anti-human T-lymphocyte immunoglobulin (ATLG) in unrelated donor transplantation. Primary endpoint was severe GvHD and relapse-free survival (SGRFS). GvHD prophylaxis consisted of ATLG and CSA/ MTX or MMF. Outcome was compared to the ATLG arm of our prospective randomized phase III multicenter trial trial (RCT) [1, 2]. 165 patients, median age 54 (18; 77) years, with haematological malignancies with early (45.5%), intermediate (17.6%), and advanced (37.0%) disease were included. ATLG dose differed between centers according to local practise (median total ATLG dose of 46 (IQR 32-60, range 15-91) mg/kg). Median follow-up was 70 months. Estimated probabilities at 5 years follow up were for SGRFS 0.27, OS 0.52, DFS 0.43, NRM 0.23, relapse 0.34, acute GvhD °III/IV 0.13, severe chronic GvHD 0.27. OS rates differed dependent on disease status. An effect of the given ATLG dose could not be separated from potential center effects. Despite higher age and more advanced disease in ATOS, outcome was similar to the ATLG arm of our RCT. This long-term, multicenter, experience in routine clinical practice confirms the GvHD-protective effect of ATLG without compromising relapse and non-relapse mortality rates.Clinical Trial Registry: German clinical trials register DRKS00004581.
© 2024. The Author(s).
Conflict of interest statement
JF and HB had received travel and lecture fees from Neovii Biotech, CS and OG have received an institutional research grant from Neovii Biotech.
Figures
References
-
- Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64. doi: 10.1016/S1470-2045(09)70225-6. - DOI - PubMed
-
- Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4:e293–e301. doi: 10.1016/S2352-3026(17)30081-9. - DOI - PubMed
-
- Baron F, Mohty M, Blaise D, Socie G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:224–34. doi: 10.3324/haematol.2016.148510. - DOI - PMC - PubMed
-
- Finke J, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Prognostic factors affecting outcome after allogeneic transplantation for hematological malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transpl. 2012;18:1716–26. doi: 10.1016/j.bbmt.2012.06.001. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

