A quantitative MRI-based approach to estimate the permeation and retention of nanomedicines in tumors
- PMID: 38493951
- PMCID: PMC11412736
- DOI: 10.1016/j.jconrel.2024.03.019
A quantitative MRI-based approach to estimate the permeation and retention of nanomedicines in tumors
Abstract
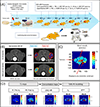
Despite the potential of the enhanced permeability and retention (EPR) effect in tumor passive targeting, many nanotherapeutics have failed to produce meaningful clinical outcomes due to the variable and challenging nature of the tumor microenvironment (TME) and EPR effect. This EPR variability across tumors and inconsistent translation of nanomedicines from preclinical to clinical settings necessitates a reliable method to assess its presence in individual tumors. This study aimed to develop a reliable and non-invasive approach to estimate the EPR effect in tumors using a clinically compatible quantitative magnetic resonance imaging (qMRI) technique combined with a nano-sized MRI contrast agent. A quantitative MR imaging was developed using a dynamic contrast-enhanced (DCE) MRI protocol. Then, the permeability and retention of the nano-sized MRI contrast agent were evaluated in three different ovarian xenograft tumor models. Results showed significant differences in EPR effects among the tumor models, with tumor growth influencing the calculated parameters of permeability (Ktrans) and retention (Ve) based on Tofts pharmacokinetic (PK) modeling. Our data indicate that the developed quantitative DCE-MRI method, combined with the Tofts PK modeling, provides a robust and non-invasive approach to screen tumors for their responsiveness to nanotherapeutics. These results imply that the developed qMRI method can be beneficial for personalized cancer treatments by ensuring that nanotherapeutics are administered only to patients with tumors showing sufficient EPR levels.
Keywords: Enhanced permeability and retention effect (EPR); MRI; Magnetic resonance imaging; Nanomedicine; Nanoparticle permeation; Tofts; Tumor vasculature leakiness.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



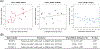
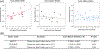

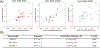
References
-
- Iwai K, Maeda H, Konno T, Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image, Cancer Research, 44 (1984) 2115–2121. - PubMed
-
- Lammers T, Kiessling F, Hennink WE, Storm G, Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress, J Control Release, 161 (2012) 175–187. - PubMed
-
- D’Mello SR, Cruz CN, Chen ML, Kapoor M, Lee SL, Tyner KM, The evolving landscape of drug products containing nanomaterials in the United States, Nat Nanotechnol, 12 (2017) 523–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

