Multimodal active subspace analysis for computing assessment oriented subspaces from neuroimaging data
- PMID: 38494061
- PMCID: PMC11100582
- DOI: 10.1016/j.jneumeth.2024.110109
Multimodal active subspace analysis for computing assessment oriented subspaces from neuroimaging data
Abstract
Background: For successful biomarker discovery, it is essential to develop computational frameworks that summarize high-dimensional neuroimaging data in terms of involved sub-systems of the brain, while also revealing underlying heterogeneous functional and structural changes covarying with specific cognitive and biological traits. However, unsupervised decompositions do not inculcate clinical assessment information, while supervised approaches extract only individual feature importance, thereby impeding qualitative interpretation at the level of subspaces.
New method: We present a novel framework to extract robust multimodal brain subspaces associated with changes in a given cognitive or biological trait. Our approach involves active subspace learning on the gradients of a trained machine learning model followed by clustering to extract and summarize the most salient and consistent subspaces associated with the target variable.
Results: Through a rigorous cross-validation procedure on an Alzheimer's disease (AD) dataset, our framework successfully extracts multimodal subspaces specific to a given clinical assessment (e.g., memory and other cognitive skills), and also retains predictive performance in standard machine learning algorithms. We also show that the salient active subspace directions occur consistently across randomly sub-sampled repetitions of the analysis.
Comparison with existing method(s): Compared to existing unsupervised decompositions based on principle component analysis, the subspace components in our framework retain higher predictive information.
Conclusions: As an important step towards biomarker discovery, our framework not only uncovers AD-related brain regions in the associated brain subspaces, but also enables automated identification of multiple underlying structural and functional sub-systems of the brain that collectively characterize changes in memory and proficiency in cognitive skills related to brain disorders like AD.
Keywords: Brain networks; Functional connectivity; Heterogeneity; Machine learning; Magnetic resonance imaging; Multimodal fusion; Neuroimaging; Subspace analysis.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Vince Calhoun reports financial support and article publishing charges were provided by the National Institutes of Health. Vince Calhoun reports financial support and article publishing charges were provided by the National Science Foundation. Ishaan Batta, Anees Abrol, and Vince Calhoun report a relationship with Georgia State University that includes: employment. Ishaan Batta, and Vince Calhoun report a relationship with the Georgia Institute of Technology that includes: employment. Vince Calhoun reports a relationship with Emory University that includes: employment.
Figures
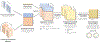




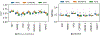
References
-
- Abavisani M. and Patel VM (2018). Deep multimodal subspace clustering networks. IEEE Journal of Selected Topics in Signal Processing, 12(6):1601–1614.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

