Promising clinical and immunological efficacy of Bacillus clausii spore probiotics for supportive treatment of persistent diarrhea in children
- PMID: 38494525
- PMCID: PMC10944834
- DOI: 10.1038/s41598-024-56627-9
Promising clinical and immunological efficacy of Bacillus clausii spore probiotics for supportive treatment of persistent diarrhea in children
Abstract
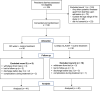
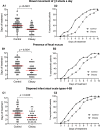
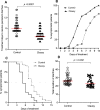
Persistent diarrhea is a severe gastroenteric disease with relatively high risk of pediatric mortality in developing countries. We conducted a randomized, double-blind, controlled clinical trial to evaluate the efficacy of liquid-form Bacillus clausii spore probiotics (LiveSpo CLAUSY; 2 billion CFU/5 mL ampoule) at high dosages of 4-6 ampoules a day in supporting treatment of children with persistent diarrhea. Our findings showed that B. clausii spores significantly improved treatment outcomes, resulting in a 2-day shorter recovery period (p < 0.05) and a 1.5-1.6 folds greater efficacy in reducing diarrhea symptoms, such as high frequency of bowel movement of ≥ 3 stools a day, presence of fecal mucus, and diapered infant stool scale types 4-5B. LiveSpo CLAUSY supportive treatment achieved 3 days (p < 0.0001) faster recovery from diarrhea disease, with 1.6-fold improved treatment efficacy. At day 5 of treatment, a significant decrease in blood levels of pro-inflammatory cytokines TNF-α, IL-17, and IL-23 by 3.24% (p = 0.0409), 29.76% (p = 0.0001), and 10.87% (p = 0.0036), respectively, was observed in the Clausy group. Simultaneously, there was a significant 37.97% decrease (p = 0.0326) in the excreted IgA in stool at day 5 in the Clausy group. Overall, the clinical study demonstrates the efficacy of B. clausii spores (LiveSpo CLAUSY) as an effective symptomatic treatment and immunomodulatory agent for persistent diarrhea in children.Trial registration: NCT05812820.
Keywords: Bacillus clausii; Cytokine; Persistent diarrhea; Probiotic; Spore.
© 2024. The Author(s).
Conflict of interest statement
LiveSpo CLAUSY is produced by LiveSpo Pharma Ltd., in which A.H.N is a founder and scientific director. The remained authors report no declarations of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

