Matching-Adjusted Indirect Comparison of Brexucabtagene Autoleucel (ZUMA-2) and Pirtobrutinib (BRUIN) in Patients with Relapsed/Refractory Mantle Cell Lymphoma Previously Treated with a Covalent Bruton Tyrosine Kinase Inhibitor
- PMID: 38494543
- PMCID: PMC11052850
- DOI: 10.1007/s12325-024-02822-z
Matching-Adjusted Indirect Comparison of Brexucabtagene Autoleucel (ZUMA-2) and Pirtobrutinib (BRUIN) in Patients with Relapsed/Refractory Mantle Cell Lymphoma Previously Treated with a Covalent Bruton Tyrosine Kinase Inhibitor
Abstract
Introduction: Patients with relapsed/refractory (R/R) mantle cell lymphoma (MCL) often require multiple lines of treatment and have a poor prognosis, particularly after failing covalent Bruton tyrosine kinase inhibitor (cBTKi) therapy. Newer treatments such as brexucabtagene autoleucel (brexu-cel, chimeric antigen receptor T cell therapy) and pirtobrutinib (non-covalent BTKi) show promise in improving outcomes.
Methods: Without direct comparative evidence, an unanchored matching-adjusted indirect comparison was conducted to estimate the relative treatment effects of brexu-cel and pirtobrutinib for post-cBTKi R/R MCL. Using logistic propensity score models, individual patient-level data from ZUMA-2 brexu-cel-infused population (N = 68) were weighted to match pre-specified clinically relevant prognostic factors based on study-level data from the BRUIN cBTKi pre-treated cohort (N = 90). The base-case model incorporated the five most pertinent factors reported in ≥ 50% of both trial populations: morphology, MCL International Prognostic Index, number of prior lines of therapy, disease stage, and prior autologous stem cell transplant. A sensitivity analysis additionally incorporated TP53 mutation and Ki-67 proliferation. Relative treatment effects were expressed as odds ratios (ORs) or hazard ratios (HRs) with 95% confidence intervals (CIs).
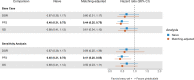
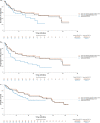
Results: In the base-case model, brexu-cel was associated with higher rates of objective response (OR 10.39 [95% CI 2.81-38.46]) and complete response (OR 10.11 [95% CI 4.26-24.00]), and improved progression-free survival (HR 0.44 [95% CI 0.25-0.75]), compared to pirtobrutinib. Overall survival and duration of response favored brexu-cel over pirtobrutinib but the differences crossed the bounds for statistical significance. Findings were consistent across the adjusted and unadjusted analyses.
Conclusions: Findings suggest that brexu-cel may offer clinically and statistically significant benefits regarding objective response, complete response, and progression-free survival compared to pirtobrutinib among patients with R/R MCL after prior cBTKi therapy. Given the short follow-up and high degree of censoring in BRUIN, an analysis incorporating updated BRUIN data may provide more definitive overall survival results.
Keywords: Brexu-cel; Brexucabtagene autoleucel; Bruton tyrosine kinase inhibitor; CAR T cell therapy; KTE-X19; MAIC; Mantle cell lymphoma; Matching-adjusted indirect comparison; Non-Hodgkin lymphoma; Pirtobrutinib.
© 2024. The Author(s).
Conflict of interest statement
Gilles Salles has received consulting fees from Abbvie, Celgene/BMS, Epizyme, Genmab, Incyte, Janssen, Kite, a Gilead Company, Loxo, Milteniy, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, and Takeda; has received payment or honoraria for speaking in symposium from Bayer, Epizyme, and Regeneron; has participated on a Data Safety Monitoring board or Advisory Board for Beigene; and has stock or stock options from Owkin. Jenny M.H. Chen, Ina Zhang, Dylan Maciel, Keith Chan, and Sam Keeping are employees of PRECISIONheor, funded by Kite, a Gilead Company for this study. Fabio Kerbauy declares no competing interests. James J. Wu is an employee of Kite, a Gilead Company; has received honoraria from the Patient-Centered Outcomes Research Institute (PCORI), both as a member of the Rare Disease Advisory Panel, and as a grants reviewer for the Improving Methods Program; has received travel/meeting support from Kite, a Gilead Company, and Amgen; owns stock in Gilead Sciences, Amgen, Abbott, AbbVie, Pfizer, Roche, Curis, Avid Biosciences, Evofem, Lensar, VBI Vaccines, and Viracta Therapeutics. Sally W. Wade has received consulting fees from Kite, a Gilead Company, Abbvie, and Johnson & Johnson. Fabio R. Kerbauy declares no competing interests. Ana Nunes is an employee of Gilead Sciences Europe; own stocks in Gilead Sciences and Amgen; and has received support for attending meetings and/or travel from Kite, a Gilead Company. Chaoling Feng is an employee of Kite, A Gilead Company; own stocks in Gilead Sciences, Boston Scientific, and has received support for attending meetings and/or travel from Kite, a Gilead Company. Ioana Kloos is an employee of Kite, A Gilead Company; own stocks in Gilead Sciences; and has been compensated for a leadership role (such as officer or member of a board of directors) for Kite, A Gilead Company. Weimin Peng is an employee of Kite, a Gilead Company; and owns stock in Gilead Sciences. Julia T. Snider is an employee of Kite, a Gilead Company; owns stock in Gilead Sciences. Bijal Shah has received consultant and education fees from Amgen, Pfizer, Novartis, BMS/Celgene/Juno, Kite, a Gilead company, Precision Biosciences, Jazz, Beigene, Adaptive, Century Therapeutics, and Autolus; and clinical trial grants from Kite, a Gilead company, Jazz, and Servier.
Figures

References
-
- American Cancer Society. Types of B-cell lymphoma 2019 [updated January 29, 2019]. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/b-cell-ly.... Accessed 2023 Aug 27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

