Molecular engineering of a spheroid-penetrating phage nanovector for photodynamic treatment of colon cancer cells
- PMID: 38494579
- PMCID: PMC10944812
- DOI: 10.1007/s00018-024-05174-7
Molecular engineering of a spheroid-penetrating phage nanovector for photodynamic treatment of colon cancer cells
Abstract
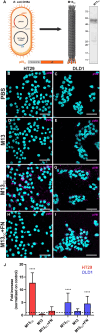
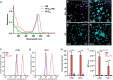
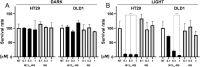
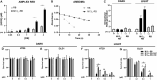
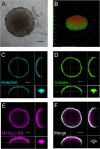
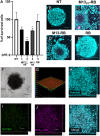
Photodynamic therapy (PDT) represents an emerging strategy to treat various malignancies, including colorectal cancer (CC), the third most common cancer type. This work presents an engineered M13 phage retargeted towards CC cells through pentavalent display of a disulfide-constrained peptide nonamer. The M13CC nanovector was conjugated with the photosensitizer Rose Bengal (RB), and the photodynamic anticancer effects of the resulting M13CC-RB bioconjugate were investigated on CC cells. We show that upon irradiation M13CC-RB is able to impair CC cell viability, and that this effect depends on i) photosensitizer concentration and ii) targeting efficiency towards CC cell lines, proving the specificity of the vector compared to unmodified M13 phage. We also demonstrate that M13CC-RB enhances generation and intracellular accumulation of reactive oxygen species (ROS) triggering CC cell death. To further investigate the anticancer potential of M13CC-RB, we performed PDT experiments on 3D CC spheroids, proving, for the first time, the ability of engineered M13 phage conjugates to deeply penetrate multicellular spheroids. Moreover, significant photodynamic effects, including spheroid disruption and cytotoxicity, were readily triggered at picomolar concentrations of the phage vector. Taken together, our results promote engineered M13 phages as promising nanovector platform for targeted photosensitization, paving the way to novel adjuvant approaches to fight CC malignancies.
Keywords: Bacteriophage; Colorectal cancer; M13; Nanovector; PDT; Spheroids.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Niculescu A-G, Grumezescu AM. Photodynamic therapy—an up-to-date review. Appl Sci. 2021;11:3626. doi: 10.3390/app11083626. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

