Application of Transthoracic Echocardiography for Cardiac Safety Evaluation in the Clinical Development Process of Vaccines Against Streptococcus pyogenes
- PMID: 38494581
- PMCID: PMC11035538
- DOI: 10.1007/s40268-024-00452-y
Application of Transthoracic Echocardiography for Cardiac Safety Evaluation in the Clinical Development Process of Vaccines Against Streptococcus pyogenes
Abstract
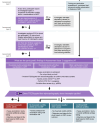
Superficial infections with Streptococcus pyogenes (Strep A), pharyngitis and impetigo can induce acute rheumatic fever, an autoimmune sequela manifesting mostly with arthritis and rheumatic carditis. Valvular heart damage can persist or advance following repeated episodes of acute rheumatic fever, causing rheumatic heart disease. Acute rheumatic fever and rheumatic heart disease disproportionately affect children and young adults in developing countries and disadvantaged communities in developed countries. People living with rheumatic heart disease are at risk of experiencing potentially fatal complications such as heart failure, bacterial endocarditis or stroke. Transthoracic echocardiography plays a central role in diagnosing both rheumatic carditis and rheumatic heart disease. Despite the obvious medical need, no licensed Strep A vaccines are currently available, as their clinical development process faces several challenges, including concerns for cardiac safety. However, the development of Strep A vaccines has been recently relaunched by many vaccine developers. In this context, a reliable and consistent safety evaluation of Strep A vaccine candidates, including the use of transthoracic echocardiography for detecting cardiac adverse events, could greatly contribute to developing a safe and efficacious product in the near future. Here, we propose a framework for the consistent use of transthoracic echocardiography to proactively detect cardiac safety events in clinical trials of Strep A vaccine candidates.
Plain language summary
Throat and skin infections caused by certain types of bacteria, named Streptococcus pyogenes, are frequent worldwide; however, in many children from less developed countries and disadvantaged communities, infections with S. pyogenes lead to a condition called acute rheumatic fever, which usually affects the joints and the heart. Damage to the heart valves may evolve to rheumatic heart disease, a permanent condition with often life-threatening complications. Rheumatic heart disease is an important health problem in places and communities where S. pyogenes infections occur frequently. A vaccine against these bacteria would help lower the number of people with valvular heart disease; however, no such vaccine exists yet. Research on vaccines against S. pyogenes was on hold for almost 30 years because of initial concerns that vaccinated children might develop acute rheumatic fever more frequently. Recently, researchers started working again on vaccines against S. pyogenes, but concerns about the safety of such vaccines persist. Doctors can reliably use echocardiography to diagnose cases of rheumatic carditis (as a sign of acute rheumatic fever) and rheumatic heart disease. Here, we propose a simple approach for the consistent use of echocardiography in clinical research of vaccines against S. pyogenes that will allow the detection of any potential heart-related side effects of the vaccine.
© 2024. GlaxoSmithKline Biologicals S.A.
Conflict of interest statement
Usman Nakakana, Alimamy Serry-Bangura, Bassey Effiom Edem, Pietro Tessitore, Leonardo Di Cesare, Danilo Gomes Moriel, Audino Podda, Iris Sarah De Ryck and Ashwani Kumar Arora are or were employees of GSK at the time of this study. Usman Nakakana, Alimamy Serry-Bangura, Danilo Gomes Moriel, Audino Podda, Iris Sarah De Ryck and Ashwani Kumar Arora hold shares in GSK. GSK is currently developing a 4-component vaccine candidate against
Figures




References
-
- Ferretti JJ, Köhler W. History of streptococcal research. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK333430/. Accessed 4 May 2022.
-
- Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. https://www.ncbi.nlm.nih.gov/books/NBK343616/. Accessed 18 May 2022.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

