Targeted intracellular delivery of dimeric STINGa by two pHLIP peptides for treatment of solid tumors
- PMID: 38495104
- PMCID: PMC10940318
- DOI: 10.3389/fphar.2024.1346756
Targeted intracellular delivery of dimeric STINGa by two pHLIP peptides for treatment of solid tumors
Abstract
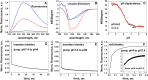
Introduction: We have developed a delivery approach that uses two pHLIP peptides that collaborate in the targeted intracellular delivery of a single payload, dimeric STINGa (dMSA). Methods: dMSA was conjugated with two pHLIP peptides via S-S cleavable self-immolating linkers to form 2pHLIP-dMSA. Results: Biophysical studies were carried out to confirm pH-triggered interactions of the 2pHLIP-dMSA with membrane lipid bilayers. The kinetics of linker self-immolation and dMSA release, the pharmacokinetics, the binding to plasma proteins, the stability of the agent in plasma, the targeting and resulting cytokine activation in tumors, and the biodistribution of the construct was investigated. This is the first study demonstrating that combining the energy of the membrane-associated folding of two pHLIPs can be utilized to enhance the targeted intracellular delivery of large therapeutic cargo payloads. Discussion: Linking two pHLIPs to the cargo extends blood half-life, and targeted delivery of dimeric STINGa induces tumor eradication and the development of robust anti-cancer immunity.
Keywords: biophysics; cold tumors; imaging; immuno-suppressive tumors; tumor acidity.
Copyright © 2024 Moshnikova, DuPont, Iraca, Klumpp, Visca, Allababidi, Pelzer, Engelman, Andreev and Reshetnyak.
Conflict of interest statement
DE, OA, and YR are founders of pHLIP, Inc., and they have shares in the company. pHLIP, Inc. sponsored synthesis and purification of dMSA and modified dMSA prepared by the Iris Biotech GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

