Mycobacterium tuberculosis Rv2617c is involved in stress response and phage infection resistance
- PMID: 38495141
- PMCID: PMC10943396
- DOI: 10.1016/j.heliyon.2024.e27400
Mycobacterium tuberculosis Rv2617c is involved in stress response and phage infection resistance
Abstract
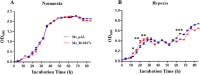
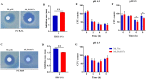
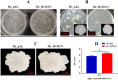
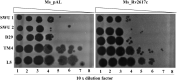
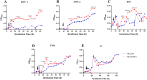
Mycobacterium tuberculosis (M. tuberculosis) is the pathogen of human tuberculosis (TB). Resistance to numerous in vivo stresses, including oxidative stress, is determinant for M. tuberculosis intracellular survival, and understanding associated mechanisms is crucial for developing new therapeutic strategies. M. tuberculosis Rv2617c has been associated with oxidative stress response when interacting with other proteins in M. tuberculosis; however, its functional promiscuity and underlying molecular mechanisms remain elusive. In this study, we investigated the phenotypic changes of Mycobacterium smegmatis (M. smegmatis) expressing Rv2617c (Ms_Rv2617c) and its behavior in the presence of various in vitro stresses and phage infections. We found that Rv2617c conferred resistance to SDS and diamide while sensitizing M. smegmatis to oxidative stress (H2O2) and altered mycobacterial phenotypic properties (single-cell clone and motility), suggestive of reprogrammed mycobacterial cell wall lipid contents exemplified by increased cell wall permeability. Interestingly, we also found that Rv2617c promoted M. smegmatis resistance to infection by phages (SWU1, SWU2, D29, and TM4) and kept phage TM4 from destroying mycobacterial biofilms. Our findings provide new insights into the role of Rv2617c in resistance to oxide and acid stresses and report for the first time on its role in phage resistance in Mycobacterium.
Keywords: DoxX domain; Mycobacteriophage resistance; Mycobacterium tuberculosis; Rv2617c; Stress responses; Virulence.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Rv2617c and P36 are virulence factors of pathogenic mycobacteria involved in resistance to oxidative stress.Virulence. 2019 Dec;10(1):1026-1033. doi: 10.1080/21505594.2019.1693714. Virulence. 2019. PMID: 31782338 Free PMC article.
-
The M. tuberculosis Rv1523 Methyltransferase Promotes Drug Resistance Through Methylation-Mediated Cell Wall Remodeling and Modulates Macrophages Immune Responses.Front Cell Infect Microbiol. 2021 Mar 12;11:622487. doi: 10.3389/fcimb.2021.622487. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33777836 Free PMC article.
-
[Frontier of mycobacterium research--host vs. mycobacterium].Kekkaku. 2005 Sep;80(9):613-29. Kekkaku. 2005. PMID: 16245793 Japanese.
-
Identification of two proteins that interact with the Erp virulence factor from Mycobacterium tuberculosis by using the bacterial two-hybrid system.BMC Mol Biol. 2009 Jan 21;10:3. doi: 10.1186/1471-2199-10-3. BMC Mol Biol. 2009. PMID: 19159459 Free PMC article.
-
In vivo efficacy of phage therapy for Mycobacterium avium infection as delivered by a nonvirulent mycobacterium.Microb Drug Resist. 2006 Spring;12(1):1-6. doi: 10.1089/mdr.2006.12.1. Microb Drug Resist. 2006. PMID: 16584300
References
-
- Yu X., et al. Mycobacterium tuberculosis PE_PGRS1 promotes mycobacteria intracellular survival via reducing the concentration of intracellular free Ca(2+) and suppressing endoplasmic reticulum stress. Mol. Immunol. 2023;154:24–32. - PubMed
LinkOut - more resources
Full Text Sources

