Neurovascular coupling impairment as a mechanism for cognitive deficits in COVID-19
- PMID: 38495306
- PMCID: PMC10943572
- DOI: 10.1093/braincomms/fcae080
Neurovascular coupling impairment as a mechanism for cognitive deficits in COVID-19
Abstract
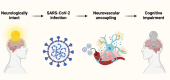
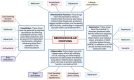
Components that comprise our brain parenchymal and cerebrovascular structures provide a homeostatic environment for proper neuronal function to ensure normal cognition. Cerebral insults (e.g. ischaemia, microbleeds and infection) alter cellular structures and physiologic processes within the neurovascular unit and contribute to cognitive dysfunction. COVID-19 has posed significant complications during acute and convalescent stages in multiple organ systems, including the brain. Cognitive impairment is a prevalent complication in COVID-19 patients, irrespective of severity of acute SARS-CoV-2 infection. Moreover, overwhelming evidence from in vitro, preclinical and clinical studies has reported SARS-CoV-2-induced pathologies in components of the neurovascular unit that are associated with cognitive impairment. Neurovascular unit disruption alters the neurovascular coupling response, a critical mechanism that regulates cerebromicrovascular blood flow to meet the energetic demands of locally active neurons. Normal cognitive processing is achieved through the neurovascular coupling response and involves the coordinated action of brain parenchymal cells (i.e. neurons and glia) and cerebrovascular cell types (i.e. endothelia, smooth muscle cells and pericytes). However, current work on COVID-19-induced cognitive impairment has yet to investigate disruption of neurovascular coupling as a causal factor. Hence, in this review, we aim to describe SARS-CoV-2's effects on the neurovascular unit and how they can impact neurovascular coupling and contribute to cognitive decline in acute and convalescent stages of the disease. Additionally, we explore potential therapeutic interventions to mitigate COVID-19-induced cognitive impairment. Given the great impact of cognitive impairment associated with COVID-19 on both individuals and public health, the necessity for a coordinated effort from fundamental scientific research to clinical application becomes imperative. This integrated endeavour is crucial for mitigating the cognitive deficits induced by COVID-19 and its subsequent burden in this especially vulnerable population.
Keywords: SARS-CoV-2; cognitive impairment; endothelium; neurovascular uncoupling; oxidative stress.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

Similar articles
-
COVID-19 Exacerbates Neurovascular Uncoupling and Contributes to Endothelial Dysfunction in Patients with Mild Cognitive Impairment.Biomolecules. 2024 Dec 18;14(12):1621. doi: 10.3390/biom14121621. Biomolecules. 2024. PMID: 39766328 Free PMC article.
-
Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline.Exp Gerontol. 2017 Aug;94:52-58. doi: 10.1016/j.exger.2016.11.004. Epub 2016 Nov 12. Exp Gerontol. 2017. PMID: 27845201 Free PMC article. Review.
-
Cerebromicrovascular mechanisms contributing to long COVID: implications for neurocognitive health.Geroscience. 2025 Feb;47(1):745-779. doi: 10.1007/s11357-024-01487-4. Epub 2025 Jan 7. Geroscience. 2025. PMID: 39777702 Free PMC article. Review.
-
Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging.Geroscience. 2019 Oct;41(5):533-542. doi: 10.1007/s11357-019-00101-2. Epub 2019 Nov 2. Geroscience. 2019. PMID: 31679124 Free PMC article.
-
Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice.Aging Cell. 2018 Apr;17(2):e12731. doi: 10.1111/acel.12731. Epub 2018 Feb 6. Aging Cell. 2018. PMID: 29405550 Free PMC article.
Cited by
-
Human herpesvirus reactivation and its potential role in the pathogenesis of post-acute sequelae of SARS-CoV-2 infection.Geroscience. 2025 Feb;47(1):167-187. doi: 10.1007/s11357-024-01323-9. Epub 2024 Aug 29. Geroscience. 2025. PMID: 39207648 Free PMC article. Review.
-
Patterns of C1-Inhibitor Plasma Levels and Kinin-Kallikrein System Activation in Relation to COVID-19 Severity.Life (Basel). 2024 Nov 21;14(12):1525. doi: 10.3390/life14121525. Life (Basel). 2024. PMID: 39768234 Free PMC article.
-
Impact of acute sleep restriction on cerebrovascular reactivity and neurovascular coupling in young men and women.J Appl Physiol (1985). 2025 Jan 1;138(1):282-288. doi: 10.1152/japplphysiol.00648.2024. Epub 2024 Dec 11. J Appl Physiol (1985). 2025. PMID: 39661323 Free PMC article.
-
COVID-19 Exacerbates Neurovascular Uncoupling and Contributes to Endothelial Dysfunction in Patients with Mild Cognitive Impairment.Biomolecules. 2024 Dec 18;14(12):1621. doi: 10.3390/biom14121621. Biomolecules. 2024. PMID: 39766328 Free PMC article.
-
Long-term neurological and cognitive impact of COVID-19: a systematic review and meta-analysis in over 4 million patients.BMC Neurol. 2025 Jun 14;25(1):250. doi: 10.1186/s12883-025-04174-9. BMC Neurol. 2025. PMID: 40514644 Free PMC article.
References
-
- WHO . WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. 2023.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
