Carbon ion irradiation combined with PD-1 inhibitor trigger abscopal effect in Lewis lung cancer via a threshold dose
- PMID: 38495488
- PMCID: PMC10937268
- DOI: 10.7150/jca.91559
Carbon ion irradiation combined with PD-1 inhibitor trigger abscopal effect in Lewis lung cancer via a threshold dose
Abstract
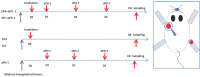
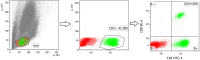
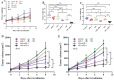
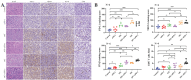
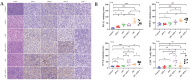
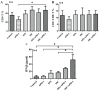
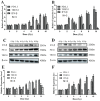
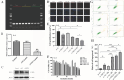
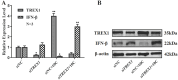
Background and goal: Carbon ion beam is radio-biologically more efficient than photons and is beneficial for treating radio-resistant tumors. Several animal experiments with tumor-bearing suggest that carbon ion beam irradiation in combination with immunotherapy yields better results, especially in controlling distant metastases. This implies that carbon ion induces a different anti-tumor immune response than photon beam. More complex molecular mechanisms need to be uncovered. This in vivo and in vitro experiment was carried out in order to examine the radio-immune effects and the mechanism of action of carbon ion beam versus X-ray in combination with PD-1 inhibitors. Methods and Materials: Lewis lung adenocarcinoma cells and C57BL/6 mice were used to create a tumor-bearing mouse model, with the non-irradiated tumor growing on the right hind leg and the irradiated tumor on the left rear. 10Gy carbon ion beam or X-ray radiation, either alone or in combination with PD-1 inhibitor, were used to treat the left back tumor. The expression of molecules linked to immunogenicity and the infiltration of CD8+ T lymphocytes into tumor tissues were both identified using immunohistochemistry. IFN-β in mouse serum was measured using an ELISA, while CD8+ T cells in mouse peripheral blood were measured using flow cytometry. Lewis cells were exposed to different dose of X-ray and carbon ion. TREX1, PD-L1, and IFN-β alterations in mRNA and protein levels were identified using Western blot or RT-PCR, respectively. TREX1 knockdown was created by siRNA transfection and exposed to various radiations. Using the CCK8 test, EdU assay, and flow cytometry, changes in cell viability, proliferation, and apoptosis rate were discovered. Results: Bilateral tumors were significantly inhibited by the use of carbon ion or X-ray in combination with PD-1, particularly to non-irradiated tumor(p<0.05). The percentage of infiltrating CD8+ T cells and the level of IFN-β expression were both raised by 10Gy carbon ion irradiation in the irradiated side tumor, although PD-L1 and TREX1 expression levels were also elevated. Lewis cell in vitro experiment further demonstrated that both X-ray and carbon ion irradiation can up-regulate the expression levels of PD-L1 and TREX1 with dose-dependent in tumors, particularly the trend of up-regulation TREX1 is more apparent at a higher dose in carbon ion, i.e. 8 or 10Gy, while the level of IFN-β is decreased. IFN-β levels were considerably raised under hypofractionated doses of carbon ion radiation by gene silencing TREX1. Conclusions: By enhancing tumor immunogenicity and increasing CD8+T infiltration in TME through a threshold dosage, X-ray or carbon ion radiation and PD-1 inhibitors improve anti-tumor activity and cause abscopal effect in Lewis lung adenocarcinoma-bearing mice. TREX1 is a possible therapeutic target and prognostic marker.
Keywords: Abscopal effect; Carbon ion; In vitro experiment; Irradiation; PD-1 inhibitor.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Microwave ablation combined with α-PD-L1 enhances abscopal effect and promotes CTL activation and intratumoral homing by a cytokine network involving IFN-γ and CXCL9.Int Immunopharmacol. 2025 Apr 24;153:114498. doi: 10.1016/j.intimp.2025.114498. Epub 2025 Mar 17. Int Immunopharmacol. 2025. PMID: 40101420
-
ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.Theranostics. 2021 Jan 19;11(7):3392-3416. doi: 10.7150/thno.52435. eCollection 2021. Theranostics. 2021. PMID: 33537094 Free PMC article.
-
α-PD-L1 mAb enhances the abscopal effect of hypo-fractionated radiation by attenuating PD-L1 expression and inducing CD8+ T-cell infiltration.Immunotherapy. 2019 Feb;11(2):101-118. doi: 10.2217/imt-2018-0049. Epub 2018 Dec 4. Immunotherapy. 2019. PMID: 30511887
-
Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action.Expert Opin Drug Deliv. 2021 Feb;18(2):187-203. doi: 10.1080/17425247.2021.1825376. Epub 2020 Oct 5. Expert Opin Drug Deliv. 2021. PMID: 32954856 Review.
-
The Effects of Gynecological Tumor Irradiation on the Immune System.Cancers (Basel). 2024 Aug 9;16(16):2804. doi: 10.3390/cancers16162804. Cancers (Basel). 2024. PMID: 39199577 Free PMC article. Review.
Cited by
-
Carbon ion irradiation mobilizes antitumor immunity: from concept to the clinic.Radiat Oncol. 2025 May 22;20(1):85. doi: 10.1186/s13014-025-02647-2. Radiat Oncol. 2025. PMID: 40405246 Free PMC article. Review.
-
The impact of 12C6 heavy ion irradiation-induced cellular mutations on the replication of the foot-and-mouth disease virus and the role of Cbr3.Cell Mol Life Sci. 2025 Jun 28;82(1):261. doi: 10.1007/s00018-025-05628-6. Cell Mol Life Sci. 2025. PMID: 40580333 Free PMC article.
-
Physical parameters and biological factors affect the abscopal effect of combining radiotherapy with immunotherapy: an update on preclinical works.Front Public Health. 2025 Jan 15;12:1517147. doi: 10.3389/fpubh.2024.1517147. eCollection 2024. Front Public Health. 2025. PMID: 39949344 Free PMC article. Review.
-
First Experiences of Pilot Clinical Studies on Boron Neutron Capture Therapy for Recurrent Gastrointestinal Cancers Using an Intravenous Injection of 10BPA.In Vivo. 2025 May-Jun;39(3):1470-1491. doi: 10.21873/invivo.13948. In Vivo. 2025. PMID: 40294997 Free PMC article.
References
-
- Geng Y, Zhang Q, Feng S, Li C, Wang L, Zhao X. et al. Safety and efficacy of combination therapy using programmed cell death protein-1/programmed cell death ligand-1 inhibitors and radiotherapy in patients with non-small-cell lung cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:1222–1239. - PMC - PubMed
-
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

