Accuracy of prenatal and postnatal biomarkers for estimating gestational age: a systematic review and meta-analysis
- PMID: 38495518
- PMCID: PMC10940947
- DOI: 10.1016/j.eclinm.2024.102498
Accuracy of prenatal and postnatal biomarkers for estimating gestational age: a systematic review and meta-analysis
Abstract
Background: Knowledge of gestational age (GA) is key in clinical management of individual obstetric patients, and critical to be able to calculate rates of preterm birth and small for GA at a population level. Currently, the gold standard for pregnancy dating is measurement of the fetal crown rump length at 11-14 weeks of gestation. However, this is not possible for women first presenting in later pregnancy, or in settings where routine ultrasound is not available. A reliable, cheap and easy to measure GA-dependent biomarker would provide an important breakthrough in estimating the age of pregnancy. Therefore, the aim of this study was to determine the accuracy of prenatal and postnatal biomarkers for estimating gestational age (GA).
Methods: Systematic review prospectively registered with PROSPERO (CRD42020167727) and reported in accordance with the PRISMA-DTA. Medline, Embase, CINAHL, LILACS, and other databases were searched from inception until September 2023 for cohort or cross-sectional studies that reported on the accuracy of prenatal and postnatal biomarkers for estimating GA. In addition, we searched Google Scholar and screened proceedings of relevant conferences and reference lists of identified studies and relevant reviews. There were no language or date restrictions. Pooled coefficients of correlation and root mean square error (RMSE, average deviation in weeks between the GA estimated by the biomarker and that estimated by the gold standard method) were calculated. The risk of bias in each included study was also assessed.
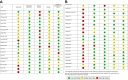
Findings: Thirty-nine studies fulfilled the inclusion criteria: 20 studies (2,050 women) assessed prenatal biomarkers (placental hormones, metabolomic profiles, proteomics, cell-free RNA transcripts, and exon-level gene expression), and 19 (1,738,652 newborns) assessed postnatal biomarkers (metabolomic profiles, DNA methylation profiles, and fetal haematological components). Among the prenatal biomarkers assessed, human chorionic gonadotrophin measured in maternal serum between 4 and 9 weeks of gestation showed the highest correlation with the reference standard GA, with a pooled coefficient of correlation of 0.88. Among the postnatal biomarkers assessed, metabolomic profiling from newborn blood spots provided the most accurate estimate of GA, with a pooled RMSE of 1.03 weeks across all GAs. It performed best for term infants with a slightly reduced accuracy for preterm or small for GA infants. The pooled RMSEs for metabolomic profiling and DNA methylation profile from cord blood samples were 1.57 and 1.60 weeks, respectively.
Interpretation: We identified no antenatal biomarkers that accurately predict GA over a wide window of pregnancy. Postnatally, metabolomic profiling from newborn blood spot provides an accurate estimate of GA, however, as this is known only after birth it is not useful to guide antenatal care. Further prenatal studies are needed to identify biomarkers that can be used in isolation, as part of a biomarker panel, or in combination with other clinical methods to narrow prediction intervals of GA estimation.
Funding: The research was funded by the Bill and Melinda Gates Foundation (INV-000368). ATP is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the NIHR Biomedical Research Centre funding scheme. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, the Department of Health, or the Department of Biotechnology. The funders of this study had no role in study design, data collection, analysis or interpretation of the data, in writing the paper or the decision to submit for publication.
Keywords: Diagnostic accuracy; Gestational age; Growth; Hormones; Metabolomics; Pregnancy; Preterm; Screening.
© 2024 The Author(s).
Conflict of interest statement
A.T.P. is a Senior Advisor of Intelligent Ultrasound. ATP is supported by the Oxford Partnership Comprehensive Biomedical Research Centre with funding from the NIHR Biomedical Research Centre (BRC) funding scheme. The views expressed herein are those of the authors and not necessarily those of the NHS, the NIHR the Department of Health or any of the other funders. All other authors declare no competing interests.
Figures
Similar articles
-
Validation of gestational age determination from ultrasound or a metabolic gestational age algorithm using exact date of conception in a cohort of newborns conceived using assisted reproduction technologies.AJOG Glob Rep. 2022 Aug 26;2(4):100091. doi: 10.1016/j.xagr.2022.100091. eCollection 2022 Nov. AJOG Glob Rep. 2022. PMID: 36536852 Free PMC article.
-
Performance of late pregnancy biometry for gestational age dating in low-income and middle-income countries: a prospective, multicountry, population-based cohort study from the WHO Alliance for Maternal and Newborn Health Improvement (AMANHI) Study Group.Lancet Glob Health. 2020 Apr;8(4):e545-e554. doi: 10.1016/S2214-109X(20)30034-6. Lancet Glob Health. 2020. PMID: 32199122 Free PMC article.
-
Assessing the diagnostic accuracy of postnatal clinical scoring methods and foot length measurement for estimating gestational age and birthweight of newborns in low- and middle-income countries: a systematic review and meta-analysis.BMJ Paediatr Open. 2024 Aug 30;8(1):e002717. doi: 10.1136/bmjpo-2024-002717. BMJ Paediatr Open. 2024. PMID: 39214548 Free PMC article.
-
Development and external validation of Indian population-specific Garbhini-GA2 model for estimating gestational age in second and third trimesters.Lancet Reg Health Southeast Asia. 2024 Feb 25;25:100362. doi: 10.1016/j.lansea.2024.100362. eCollection 2024 Jun. Lancet Reg Health Southeast Asia. 2024. PMID: 39021476 Free PMC article.
-
Three biomarker tests to help diagnose preterm labour: a systematic review and economic evaluation.Health Technol Assess. 2019 Mar;23(13):1-226. doi: 10.3310/hta23130. Health Technol Assess. 2019. PMID: 30917097 Free PMC article.
Cited by
-
Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment.Metabolites. 2024 May 7;14(5):269. doi: 10.3390/metabo14050269. Metabolites. 2024. PMID: 38786746 Free PMC article. Review.
References
-
- Lawn J.E., Ohuma E.O., Bradley E., et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401(10389):1707–1719. - PubMed
-
- Savitz D.A., Terry J.W., Jr., Dole N., Thorp J.M., Jr., Siega-Riz A.M., Herring A.H. Comparison of pregnancy dating by last menstrual period, ultrasound scanning, and their combination. Am J Obstet Gynecol. 2002;187(6):1660–1666. - PubMed
-
- Napolitano R., Dhami J., Ohuma E.O., et al. Pregnancy dating by fetal crown-rump length: a systematic review of charts. BJOG. 2014;121(5):556–565. - PubMed
-
- Brighton A., D'Arcy R., Kirtley S., Kennedy S. Perceptions of prenatal and obstetric care in Sub-Saharan Africa. Int J Gynaecol Obstet. 2013;120(3):224–227. - PubMed
LinkOut - more resources
Full Text Sources