SARS-CoV-2 recombinase polymerase amplification assay with lateral flow readout and duplexed full process internal control
- PMID: 38495597
- PMCID: PMC10939122
- DOI: 10.1039/d3sd00246b
SARS-CoV-2 recombinase polymerase amplification assay with lateral flow readout and duplexed full process internal control
Abstract
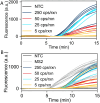
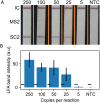
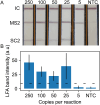
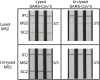
Nucleic acid amplification tests for the detection of SARS-CoV-2 have been an important testing mechanism for the COVID-19 pandemic. While these traditional nucleic acid diagnostic methods are highly sensitive and selective, they are not suited to home or clinic-based uses. Comparatively, rapid antigen tests are cost-effective and user friendly but lack in sensitivity and specificity. Here we report on the development of a one-pot, duplexed reverse transcriptase recombinase polymerase amplification SARS-CoV-2 assay with MS2 bacteriophage as a full process control. Detection is carried out with either real-time fluorescence or lateral flow readout with an analytical sensitivity of 50 copies per reaction. Unlike previously published assays, the RNA-based MS2 bacteriophage control reports on successful operation of lysis, reverse transcription, and amplification. This SARS-CoV-2 assay features highly sensitive detection, visual readout through an LFA strip, results in less than 25 minutes, minimal instrumentation, and a useful process internal control to rule out false negative test results.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Brihn A. Chang J. OYong K. Balter S. Terashita D. Rubin Z. Yeganeh N. Diagnostic Performance of an Antigen Test with RT-PCR for the Detection of SARS-CoV-2 in a Hospital Setting — Los Angeles County, California, June–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70(19):702–706. doi: 10.15585/mmwr.mm7019a3. doi: 10.15585/mmwr.mm7019a3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
