Modifying miRs for effective reprogramming of fibroblasts to cardiomyocytes
- PMID: 38495845
- PMCID: PMC10943962
- DOI: 10.1016/j.omtn.2024.102160
Modifying miRs for effective reprogramming of fibroblasts to cardiomyocytes
Abstract
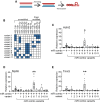
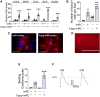
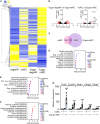
Reprogramming scar fibroblasts into cardiomyocytes has been proposed to reverse the damage associated with myocardial infarction. However, the limited improvement in cardiac function calls for enhanced strategies. We reported enhanced efficacy of our miR reprogramming cocktail miR combo (miR-1, miR-133a, miR-208a, and miR-499) via RNA-sensing receptor stimulation. We hypothesized that we could combine RNA-sensing receptor activation with fibroblast reprogramming by chemically modifying miR combo. To test the hypothesis, miR combo was modified to enhance interaction with the RNA-sensing receptor Rig1 via the addition of a 5'-triphosphate (5'ppp) group. Importantly, when compared with unmodified miR combo, 5'ppp-modified miR combo markedly improved reprogramming efficacy in vitro. Enhanced reprogramming efficacy correlated with a type-I interferon immune response with strong and selective secretion of interferon β (IFNβ). Antibody blocking studies and media replacement experiments indicated that 5'ppp-miR combo utilized IFNβ to enhance fibroblast reprogramming efficacy. In conclusion, miRs can acquire powerful additional roles through chemical modification that potentially increases their clinical applications.
Keywords: 5′-triphosphorylation; IFNβ; MT: Non-coding RNAs; RNA modification; cardiomyocytes; fibroblasts; innate immune signaling; miRs; reprogramming.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Conservation of miR combo based direct cardiac reprogramming.Biochem Biophys Rep. 2022 Jul 13;31:101310. doi: 10.1016/j.bbrep.2022.101310. eCollection 2022 Sep. Biochem Biophys Rep. 2022. PMID: 35860436 Free PMC article.
-
Demethylation of H3K27 Is Essential for the Induction of Direct Cardiac Reprogramming by miR Combo.Circ Res. 2017 Apr 28;120(9):1403-1413. doi: 10.1161/CIRCRESAHA.116.308741. Epub 2017 Feb 16. Circ Res. 2017. PMID: 28209718 Free PMC article.
-
Tissue-engineered 3-dimensional (3D) microenvironment enhances the direct reprogramming of fibroblasts into cardiomyocytes by microRNAs.Sci Rep. 2016 Dec 12;6:38815. doi: 10.1038/srep38815. Sci Rep. 2016. PMID: 27941896 Free PMC article.
-
Molecular discoveries and treatment strategies by direct reprogramming in cardiac regeneration.Transl Res. 2019 Jan;203:73-87. doi: 10.1016/j.trsl.2018.07.012. Epub 2018 Jul 31. Transl Res. 2019. PMID: 30142308 Free PMC article. Review.
-
Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction).J Mol Cell Cardiol. 2016 May;94:107-121. doi: 10.1016/j.yjmcc.2016.03.015. Epub 2016 Apr 4. J Mol Cell Cardiol. 2016. PMID: 27056419 Review.
Cited by
-
Nucleosome repositioning in cardiac reprogramming.PLoS One. 2025 Jan 15;20(1):e0317718. doi: 10.1371/journal.pone.0317718. eCollection 2025. PLoS One. 2025. PMID: 39813277 Free PMC article.
References
LinkOut - more resources
Full Text Sources

