Integrating 40-GEP Testing to Improve Clinical Recommendations for Adjuvant Radiation for Cutaneous Squamous Cell Carcinoma: Multidisciplinary Consensus Guidelines
- PMID: 38495846
- PMCID: PMC10939503
Integrating 40-GEP Testing to Improve Clinical Recommendations for Adjuvant Radiation for Cutaneous Squamous Cell Carcinoma: Multidisciplinary Consensus Guidelines
Abstract
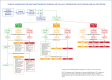
Early identification and intervention in patients with cutaneous squamous cell carcinoma (cSCC) who are at high risk for metastasis is important for optimal outcomes. Prognostic tools (e.g., American Joint Committee on Cancer, 8th edition [AJCC-8]) and management guidelines (National Comprehensive Cancer Network® [NCCN]) are useful in helping to identify high-risk patients with cSCC who might benefit from adjuvant therapies, such as radiation and/or immunotherapies; however, traditional staging and management guidelines rely on clinicopathologic risk factors to predict risk, which limits their prognostic accuracy. Gene expression profiling (GEP) is a clinically available, objective metric that can be used in conjunction with traditional clinicopathological staging to help clinicians stratify risk in patients with cSCC. The validated 40-GEP test can accurately classify patients with at least one high-risk feature as being at low (Class 1), higher (Class 2A), or highest (Class 2B) biological risk of nodal or distant metastasis within three years of diagnosis. A multidisciplinary panel comprising radiation oncologists and dermatologists/Mohs micrographic surgeons with expertise in cSCC management convened in June 2023 to discuss the utility of 40-GEP testing in cSCC clinical decision-making in regard to adjuvant radiation therapy (ART). The panel identified gaps in clinical practice in which 40-GEP testing has particular utility: in escalation of care for lower-stage patients with high-risk tumors; in de-escalation of care for patients for whom the risks of ART may outweigh the benefits; and in decision-making regarding elective radiation to the nodal basin. The expert panel developed a risk-based clinical workflow for ART in patients with cSCC, utilizing 40-GEP testing within NCCN management guidelines and AJCC-8 staging.
Keywords: 40-GEP; adjuvant radiation therapy; consensus; cutaneous squamous cell carcinoma; gene expression profiling; prognosis; risk of recurrence; risk stratification.
Copyright © 2024. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
DISCLOSURES: All authors received an honorarium from for their participation in this roundtable supplement. Dr. Tolkachjov is a speaker and investigator for Castle Biosciences, Inc., and Bioventus/LifeNet. Dr. Marquardt has served as a speaker for Castle Biosciences, Inc.
Figures

References
-
- Amin MB, Edge SB, Greene FL, editors. American Joint Commission on Cancer Staging Manual (8th ed). 2017. Switzerland: Springer.
-
- https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Squamous cell skin cancer, V1.2024. 9 Nov 2023. Accessed 18 Dec 2023.
-
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149(4):402–410. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
