Engineering customized nanovaccines for enhanced cancer immunotherapy
- PMID: 38496036
- PMCID: PMC10940734
- DOI: 10.1016/j.bioactmat.2024.02.028
Engineering customized nanovaccines for enhanced cancer immunotherapy
Abstract
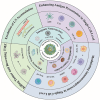
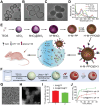
Nanovaccines have gathered significant attention for their potential to elicit tumor-specific immunological responses. Despite notable progress in tumor immunotherapy, nanovaccines still encounter considerable challenges such as low delivery efficiency, limited targeting ability, and suboptimal efficacy. With an aim of addressing these issues, engineering customized nanovaccines through modification or functionalization has emerged as a promising approach. These tailored nanovaccines not only enhance antigen presentation, but also effectively modulate immunosuppression within the tumor microenvironment. Specifically, they are distinguished by their diverse sizes, shapes, charges, structures, and unique physicochemical properties, along with targeting ligands. These features of nanovaccines facilitate lymph node accumulation and activation/regulation of immune cells. This overview of bespoke nanovaccines underscores their potential in both prophylactic and therapeutic applications, offering insights into their future development and role in cancer immunotherapy.
Keywords: Customized structure; Enhanced cancer immunotherapy; Nanovaccines; Prophylactic and therapeutic applications; Tailored-ligand.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Nam J., Son S., Park K.S., et al. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019;(4):398–414.
-
- Ribas A., Haining W.N., Schumacher T.N.M. When cancer cells become the enablers of an antitumor immune response. Cancer Discov. 2022;(12):2244–2248. - PubMed
-
- Schuster M., Nechansky A., Kircheis R. Cancer immunotherapy. Biotechnol. J.: Healthcare, Nutrition, Technology. 2006;(1):138–147. - PubMed
-
- Saxena M., van der Burg S.H., Melief C.J.M., et al. Therapeutic cancer vaccines. Nat. Rev. Cancer. 2021;(21):360–378. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

