Precursor- and waste-free synthesis of spark-ablated nanoparticles with enhanced photocatalytic activity and stability towards airborne organic pollutant degradation
- PMID: 38496350
- PMCID: PMC10939172
- DOI: 10.1039/d3en00348e
Precursor- and waste-free synthesis of spark-ablated nanoparticles with enhanced photocatalytic activity and stability towards airborne organic pollutant degradation
Abstract
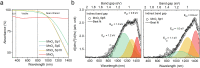
Photocatalyst synthesis typically involves multiple steps, expensive precursors, and solvents. In contrast, spark ablation offers a simple process of electrical discharges in a gap between two electrodes made from a desirable material. This enables a precursor- and waste-free generation of pure metal oxide nanoparticles or mixtures of various compositions. This study presents a two-step method for the production of photocatalytic filters with deposited airborne MnOx, TiO2, and ZnO nanoparticles using spark ablation and calcination processes. The resulting MnOx and TiO2 filters demonstrated almost twice the activity with outstanding performance stability, as compared to sol-gel MnO2 and commercial TiO2. The introduced method is not only simple, precursor- and waste-free, and leads to superior performance for the case studied, but it also has future potential due to its versatility. It can easily produce mixed and doped materials with further improved properties, making it an interesting avenue for future research.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







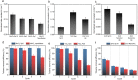
References
-
- Alberici R. M. Jardim W. F. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal., B. 1997;14:55–68. doi: 10.1016/S0926-3373(97)00012-X. - DOI
-
- Yang C. Miao G. Pi Y. Xia Q. Wu J. Li Z. Xiao J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019;370:1128–1153. doi: 10.1016/j.cej.2019.03.232. - DOI
-
- Yaghoubi H. Li Z. Chen Y. Ngo H. T. Bhethanabotla V. R. Joseph B. Ma S. Schlaf R. Takshi A. Toward a Visible Light-Driven Photocatalyst: The Effect of Midgap-States-Induced Energy Gap of Undoped TiO2 Nanoparticles. ACS Catal. 2015;5:327–335. doi: 10.1021/cs501539q. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
