This is a preprint.
Improved synthesis and application of an alkyne-functionalized isoprenoid analogue to study the prenylomes of motor neurons, astrocytes and their stem cell progenitors
- PMID: 38496415
- PMCID: PMC10942399
- DOI: 10.1101/2024.03.03.583211
Improved synthesis and application of an alkyne-functionalized isoprenoid analogue to study the prenylomes of motor neurons, astrocytes and their stem cell progenitors
Update in
-
Improved synthesis and application of an alkyne-functionalized isoprenoid analogue to study the prenylomes of motor neurons, astrocytes and their stem cell progenitors.Bioorg Chem. 2024 Jun;147:107365. doi: 10.1016/j.bioorg.2024.107365. Epub 2024 Apr 16. Bioorg Chem. 2024. PMID: 38636436 Free PMC article.
Abstract
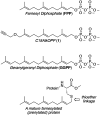
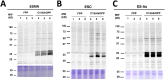
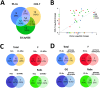
Protein prenylation is one example of a broad class of post-translational modifications where proteins are covalently linked to various hydrophobic moieties. To globally identify and monitor levels of all prenylated proteins in a cell simultaneously, our laboratory and others have developed chemical proteomic approaches that rely on the metabolic incorporation of isoprenoid analogues bearing bio-orthogonal functionality followed by enrichment and subsequent quantitative proteomic analysis. Here, several improvements in the synthesis of the alkyne-containing isoprenoid analogue C15AlkOPP are reported to improve synthetic efficiency. Next, metabolic labeling with C15AlkOPP was optimized to obtain useful levels of metabolic incorporation of the probe in several types of primary cells. Those conditions were then used to study the prenylomes of motor neurons (ES-MNs), astrocytes (ES-As), and their embryonic stem cell progenitors (ESCs), which allowed for the identification of 54 prenylated proteins from ESCs, 50 from ES-MNs and 84 from ES-As, representing all types of prenylation. Bioinformatic analysis revealed specific enriched pathways, including nervous system development, chemokine signaling, Rho GTPase signaling, and adhesion. Hierarchical clustering showed that most enriched pathways in all three cell types are related to GTPase activity and vesicular transport. In contrast, STRING analysis showed significant interactions in two populations that appear to be cell type dependent. The data provided herein demonstrates that robust incorporation of C15AlkOPP can be obtained in ES-MNs and related primary cells purified via magnetic-activated cell sorting allowing the identification and quantification of numerous prenylated proteins. These results suggest that metabolic labeling with C15AlkOPP should be an effective approach for investigating the role of prenylated proteins in primary cells in both normal cells and disease pathologies, including ALS.
Figures





References
-
- Zhang F. L.; Casey P. J. Protein Prenylation: Molecular Mechanisms and Functional Consequences. Annu. Rev. Biochem. 1996, 65 (1), 241–269. - PubMed
-
- Slater H. FDA Grants Fast Track Designation to Tipifarnib for the Treatment of Patients with HNSCC https://www.cancernetwork.com/view/fda-grants-fast-track-designation-tip... (accessed Feb 3, 2024).
-
- Wang Y.; Xu F.; Nichols C. B.; Shi Y.; Hellinga H. W.; Alspaugh J. A.; Distefano M. D.; Beese L. S. Structure-Guided Discovery of Potent Antifungals That Prevent Ras Signaling by Inhibiting Protein Farnesyltransferase. J. Med. Chem. 2022, 65 (20), 13753–13770. 10.1021/acs.jmedchem.2c00902. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
