This is a preprint.
Salmonella Typhimurium infection inhibits macrophage IFNβ signaling in a TLR4-dependent manner
- PMID: 38496427
- PMCID: PMC10942315
- DOI: 10.1101/2024.03.05.583530
Salmonella Typhimurium infection inhibits macrophage IFNβ signaling in a TLR4-dependent manner
Update in
-
Salmonella Typhimurium infection inhibits macrophage IFNβ signaling in a TLR4-dependent manner.Infect Immun. 2024 Oct 15;92(10):e0009824. doi: 10.1128/iai.00098-24. Epub 2024 Sep 13. Infect Immun. 2024. PMID: 39269166 Free PMC article.
Abstract
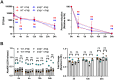
Type I Interferons (IFNs) generally have a protective role during viral infections, but their function during bacterial infections is dependent on the bacterial species. Legionella pneumophila, Shigella sonnei and Mycobacterium tuberculosis can inhibit type I IFN signaling. Here we examined the role of type I IFN, specifically IFNβ, in the context of Salmonella enterica serovar Typhimurium (STm) macrophage infections and the capacity of STm to inhibit type I IFN signaling. We demonstrate that IFNβ has no effect on the intracellular growth of STm in infected bone marrow derived macrophages (BMDMs) derived from C57BL/6 mice. STm infection inhibits IFNβ signaling but not IFNγ signaling in a murine macrophage cell line. We show that this inhibition is independent of the type III and type VI secretion systems expressed by STm and is also independent of bacterial phagocytosis. The inhibition is Toll-like receptor 4 (TLR4)-dependent as the TLR4 ligand, lipopolysaccharide (LPS), alone is sufficient to inhibit IFNβ-mediated signaling and STm-infected, TLR4-deficient BMDMs do not exhibit inhibited IFNβ signaling. In summary, we show that macrophages exposed to STm have reduced IFNβ signaling via crosstalk with TLR4 signaling, and that IFNβ signaling does not affect cell autonomous host defense against STm.
Figures





References
-
- Hapfelmeier S, Stecher B, Barthel M, et al. The Salmonella Pathogenicity Island (SPI)-2 and SPI-1 Type III Secretion Systems Allow Salmonella Serovar typhimurium to Trigger Colitis via MyD88-Dependent and MyD88-Independent Mechanisms1. The Journal of Immunology. 2005;174(3):1675–1685. doi:10.4049/jimmunol.174.3.1675 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
