This is a preprint.
Molecular Mimicry as a Mechanism of Viral Immune Evasion and Autoimmunity
- PMID: 38496443
- PMCID: PMC10942439
- DOI: 10.1101/2024.03.08.583134
Molecular Mimicry as a Mechanism of Viral Immune Evasion and Autoimmunity
Update in
-
Molecular mimicry as a mechanism of viral immune evasion and autoimmunity.Nat Commun. 2024 Oct 30;15(1):9403. doi: 10.1038/s41467-024-53658-8. Nat Commun. 2024. PMID: 39477943 Free PMC article.
Abstract
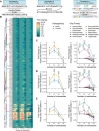
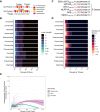
Mimicry of host protein structures ("molecular mimicry") is a common mechanism employed by viruses to evade the host's immune system. To date, studies have primarily evaluated molecular mimicry in the context of full protein structural mimics. However, recent work has demonstrated that short linear amino acid (AA) molecular mimics can elicit cross-reactive antibodies and T-cells from the host, which may contribute to development and progression of autoimmunity. Despite this, the prevalence of molecular mimics throughout the human virome has not been fully explored. In this study, we evaluate 134 human infecting viruses and find significant usage of linear mimicry across the virome, particularly those in the herpesviridae and poxviridae families. Furthermore, we identify that proteins involved in cellular replication and inflammation, those expressed from autosomes, the X chromosome, and in thymic cells are over-enriched in viral mimicry. Finally, we demonstrate that short linear mimicry from Epstein-Barr virus (EBV) is significantly higher in auto-antibodies found in multiple sclerosis patients to a greater degree than previously appreciated. Our results demonstrate that human-infecting viruses frequently leverage mimicry in the course of their infection, point to substantial evolutionary pressure for mimicry, and highlight mimicry's important role in human autoimmunity. Clinically, our findings could translate to development of novel therapeutic strategies that target viral infections linked to autoimmunity, with the goal of eliminating disease-associated latent viruses and preventing their reactivation.
Conflict of interest statement
Ethics declarations / Competing interests CM, CW, AR, CF, BM, NL, and DW have nothing to disclose. EM has received research funding from Babson Diagnostics, honorarium from Multiple Sclerosis Association of America and has served on advisory boards of Genentech, Horizon, Teva and Viela Bio.
Figures



Similar articles
-
Molecular mimicry as a mechanism of viral immune evasion and autoimmunity.Nat Commun. 2024 Oct 30;15(1):9403. doi: 10.1038/s41467-024-53658-8. Nat Commun. 2024. PMID: 39477943 Free PMC article.
-
Epstein-Barr Functional Mimicry: Pathogenicity of Oncogenic Latent Membrane Protein-1 in Systemic Lupus Erythematosus and Autoimmunity.Front Immunol. 2021 Feb 3;11:606936. doi: 10.3389/fimmu.2020.606936. eCollection 2020. Front Immunol. 2021. PMID: 33613527 Free PMC article.
-
Epstein-Barr virus and multiple sclerosis.Curr Neurol Neurosci Rep. 2007 May;7(3):253-8. doi: 10.1007/s11910-007-0038-y. Curr Neurol Neurosci Rep. 2007. PMID: 17488592 Review.
-
Cumulative Roles for Epstein-Barr Virus, Human Endogenous Retroviruses, and Human Herpes Virus-6 in Driving an Inflammatory Cascade Underlying MS Pathogenesis.Front Immunol. 2021 Nov 1;12:757302. doi: 10.3389/fimmu.2021.757302. eCollection 2021. Front Immunol. 2021. PMID: 34790199 Free PMC article.
-
Molecular mimicry of host short linear motif-mediated interactions utilised by viruses for entry.Mol Biol Rep. 2023 May;50(5):4665-4673. doi: 10.1007/s11033-023-08389-2. Epub 2023 Apr 4. Mol Biol Rep. 2023. PMID: 37016039 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
