This is a preprint.
The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm
- PMID: 38496535
- PMCID: PMC10942401
- DOI: 10.1101/2024.03.04.583310
The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm
Update in
-
The sodium-proton exchangers sNHE and NHE1 control plasma membrane hyperpolarization in mouse sperm.J Biol Chem. 2024 Dec;300(12):107932. doi: 10.1016/j.jbc.2024.107932. Epub 2024 Oct 28. J Biol Chem. 2024. PMID: 39476963 Free PMC article.
Abstract
Sperm capacitation, crucial for fertilization, occurs in the female reproductive tract and can be replicated in vitro using a medium rich in bicarbonate, calcium, and albumin. These components trigger the cAMP-PKA signaling cascade, proposed to promote hyperpolarization of the mouse sperm plasma membrane through activation of SLO3 K+ channel. Hyperpolarization is a hallmark of capacitation: proper membrane hyperpolarization renders higher in vitro fertilizing ability, while Slo3 KO mice are infertile. However, the precise regulation of SLO3 opening remains elusive. Our study challenges the involvement of PKA in this event and reveals the role of Na+/H+ exchangers. During capacitation, calcium increase through CatSper channels activates NHE1, while cAMP directly stimulates the sperm-specific NHE, collectively promoting the alkalinization threshold needed for SLO3 opening. Hyperpolarization then feeds back Na+/H+ activity. Our work is supported by pharmacology, and a plethora of KO mouse models, and proposes a novel pathway leading to hyperpolarization.
Conflict of interest statement
Competing interest Authors declare no competing interests.
Figures

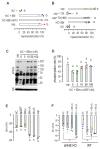
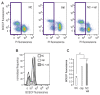

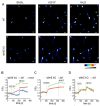
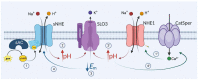
References
-
- Stival C. et al. Sperm capacitation and acrosome reaction in mammalian sperm. Adv Anat Embryol Cell Biol 220, 93–106 (2016). - PubMed
-
- Matamoros-Volante A. & Trevino C. L. Capacitation-associated alkalization in human sperm is differentially controlled at the subcellular level. J Cell Sci 133, (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
