This is a preprint.
CD47 is Required for Mesenchymal Progenitor Proliferation and Fracture Repair
- PMID: 38496546
- PMCID: PMC10942414
- DOI: 10.1101/2024.03.06.583756
CD47 is Required for Mesenchymal Progenitor Proliferation and Fracture Repair
Update in
-
CD47 is required for mesenchymal progenitor proliferation and fracture repair.Bone Res. 2025 Mar 3;13(1):29. doi: 10.1038/s41413-025-00409-0. Bone Res. 2025. PMID: 40025005 Free PMC article.
Abstract
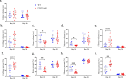
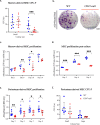
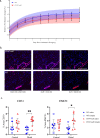
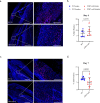
CD47 is a ubiquitous and pleiotropic cell-surface receptor. Disrupting CD47 enhances injury repair in various tissues but the role of CD47 has not been studied in bone injuries. In a murine closed-fracture model, CD47-null mice showed decreased callus bone volume, bone mineral content, and tissue mineral content as assessed by microcomputed tomography 10 days post-fracture, and increased fibrous volume as determined by histology. To understand the cellular basis for this phenotype, mesenchymal progenitors (MSC) were harvested from bone marrow. CD47-null MSC showed decreased large fibroblast colony formation (CFU-F), significantly less proliferation, and fewer cells in S-phase, although osteoblast differentiation was unaffected. However, consistent with prior research, CD47-null endothelial cells showed increased proliferation relative to WT cells. Similarly, in a murine ischemic fracture model, CD47-null mice showed reduced fracture callus bone volume and bone mineral content relative to WT. Consistent with our in vitro results, in vivo EdU labeling showed decreased cell proliferation in the callus of CD47-null mice, while staining for CD31 and endomucin demonstrated increased endothelial cell mass. Finally, WT mice administered a CD47 morpholino, which blocks CD47 protein production, showed a callus phenotype similar to that of non-ischemic and ischemic fractures in CD47-null mice, suggesting the phenotype was not due to developmental changes in the knockout mice. Thus, inhibition of CD47 during bone healing reduces both non-ischemic and ischemic fracture healing, in part, by decreasing MSC proliferation. Furthermore, the increase in endothelial cell proliferation and early blood vessel density caused by CD47 disruption is not sufficient to overcome MSC dysfunction.
Keywords: CD47; Mesenchymal progenitor cell; angiogenesis; callus; fracture healing.
Conflict of interest statement
Conflict of Interests The authors do not have any conflicts of interest to disclose.
Figures




References
-
- Lindberg F. P., Gresham H. D., Schwarz E. & Brown E. J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol 123, 485–496 (1993). 10.1083/jcb.123.2.485 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
