This is a preprint.
Comprehensive analysis of CXXX sequence space reveals that S. cerevisiae GGTase-I mainly relies on a2X substrate determinants
- PMID: 38496651
- PMCID: PMC10942308
- DOI: 10.1101/2024.03.04.583369
Comprehensive analysis of CXXX sequence space reveals that S. cerevisiae GGTase-I mainly relies on a2X substrate determinants
Update in
-
Comprehensive analysis of CXXX sequence space reveals that Saccharomyces cerevisiae GGTase-I mainly relies on a2X substrate determinants.G3 (Bethesda). 2024 Aug 7;14(8):jkae121. doi: 10.1093/g3journal/jkae121. G3 (Bethesda). 2024. PMID: 38839053 Free PMC article.
Abstract
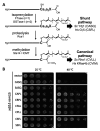
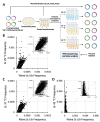
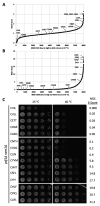
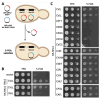
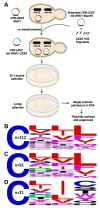
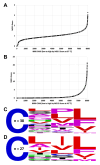
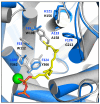
Many proteins undergo a post-translational lipid attachment, which increases their hydrophobicity, thus strengthening their membrane association properties or aiding in protein interactions. Geranylgeranyltransferase-I (GGTase-I) is an enzyme involved in a three-step post-translational modification (PTM) pathway that attaches a 20-carbon lipid group called geranylgeranyl at the carboxy-terminal cysteine of proteins ending in a canonical CaaL motif (C - cysteine, a - aliphatic, L - often leucine, but can be phenylalanine, isoleucine, methionine, or valine). Genetic approaches involving two distinct reporters were employed in this study to assess S. cerevisiae GGTase-I specificity, for which limited data exists, towards all 8000 CXXX combinations. Orthogonal biochemical analyses and structure-based alignments were also performed to better understand the features required for optimal target interaction. These approaches indicate that yeast GGTase-I best modifies the Cxa[L/F/I/M/V] sequence that resembles but is not an exact match for the canonical CaaL motif. We also observed that minor modification of non-canonical sequences is possible. A consistent feature associated with well-modified sequences was the presence of a non-polar a2 residue and a hydrophobic terminal residue, which are features recognized by mammalian GGTase-I. These results thus support that mammalian and yeast GGTase-I exhibit considerable shared specificity.
Keywords: genetic screen; geranylgeranyltransferase-I; next-generation sequencing; target specificity.
Conflict of interest statement
Conflict of interest The authors have declared no competing interests exist.
Figures


Similar articles
-
Comprehensive analysis of CXXX sequence space reveals that Saccharomyces cerevisiae GGTase-I mainly relies on a2X substrate determinants.G3 (Bethesda). 2024 Aug 7;14(8):jkae121. doi: 10.1093/g3journal/jkae121. G3 (Bethesda). 2024. PMID: 38839053 Free PMC article.
-
Genetic evidence for in vivo cross-specificity of the CaaX-box protein prenyltransferases farnesyltransferase and geranylgeranyltransferase-I in Saccharomyces cerevisiae.Mol Cell Biol. 1993 Jul;13(7):4260-75. doi: 10.1128/mcb.13.7.4260-4275.1993. Mol Cell Biol. 1993. PMID: 8321228 Free PMC article.
-
Sequence dependence of protein isoprenylation.J Biol Chem. 1991 Aug 5;266(22):14603-10. J Biol Chem. 1991. PMID: 1860864
-
[Protein farnesyl and geranylgeranyl transferases].C R Seances Soc Biol Fil. 1991;185(5):290-305. C R Seances Soc Biol Fil. 1991. PMID: 1806188 Review. French.
-
Protein Prenyltransferases and Their Inhibitors: Structural and Functional Characterization.Int J Mol Sci. 2022 May 12;23(10):5424. doi: 10.3390/ijms23105424. Int J Mol Sci. 2022. PMID: 35628237 Free PMC article. Review.
References
-
- Benetka W., Koranda M. and Eisenhaber F. (2006). “Protein Prenylation: An (Almost) Comprehensive Overview on Discovery History, Enzymology, and Significance in Physiology and Disease.” Monatshefte für Chemie / Chemical Monthly 137: 1241–1281.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
