This is a preprint.
Sarm1 is not necessary for activation of neuron-intrinsic growth programs yet required for the Schwann cell repair response and peripheral nerve regeneration
- PMID: 38496662
- PMCID: PMC10942360
- DOI: 10.1101/2024.03.04.583374
Sarm1 is not necessary for activation of neuron-intrinsic growth programs yet required for the Schwann cell repair response and peripheral nerve regeneration
Abstract
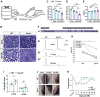
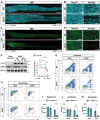
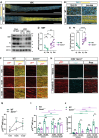
Upon peripheral nervous system (PNS) injury, severed axons undergo rapid SARM1-dependent Wallerian degeneration (WD). In mammals, the role of SARM1 in PNS regeneration, however, is unknown. Here we demonstrate that Sarm1 is not required for axotomy induced activation of neuron-intrinsic growth programs and axonal growth into a nerve crush site. However, in the distal nerve, Sarm1 is necessary for the timely induction of the Schwann cell (SC) repair response, nerve inflammation, myelin clearance, and regeneration of sensory and motor axons. In Sarm1-/- mice, regenerated fibers exhibit reduced axon caliber, defective nerve conduction, and recovery of motor function is delayed. The growth hostile environment of Sarm1-/- distal nerve tissue was demonstrated by grafting of Sarm1-/- nerve into WT recipients. SC lineage tracing in injured WT and Sarm1-/- mice revealed morphological differences. In the Sarm1-/- distal nerve, the appearance of p75NTR+, c-Jun+ SCs is significantly delayed. Ex vivo, p75NTR and c-Jun upregulation in Sarm1-/- nerves can be rescued by pharmacological inhibition of ErbB kinase. Together, our studies show that Sarm1 is not necessary for the activation of neuron intrinsic growth programs but in the distal nerve is required for the orchestration of cellular programs that underlie rapid axon extension.
Figures






References
-
- Arthur-Farraj P. J., Latouche M., Wilton D. K., Quintes S., Chabrol E., Banerjee A., Woodhoo A., Jenkins B., Rahman M., Turmaine M., Wicher G. K., Mitter R., Greensmith L., Behrens A., Raivich G., Mirsky R., and Jessen K. R.. 2012. ‘c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration’, Neuron, 75: 633–47. - PMC - PubMed
-
- Bastien D., and Lacroix S.. 2014. ‘Cytokine pathways regulating glial and leukocyte function after spinal cord and peripheral nerve injury’, Exp Neurol, 258: 62–77. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
