Does dose reduction of afatinib affect treatment outcomes of patients with EGFR-mutant metastatic non-small cell lung cancer in real-world clinical practice?
- PMID: 38496703
- PMCID: PMC10938108
- DOI: 10.21037/tlcr-23-691
Does dose reduction of afatinib affect treatment outcomes of patients with EGFR-mutant metastatic non-small cell lung cancer in real-world clinical practice?
Abstract
Background: Afatinib can be started at a dose lower than the recommended starting dose of 40 mg/day for the treatment of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC), however treatment outcomes in real-world clinical practice remains unclear.
Methods: This retrospective study of patients with NSCLC from 18 major hospitals (public, private or university teaching hospitals) enrolled in Malaysia's National Cardiovascular and Thoracic Surgical Database (NCTSD) assessed the efficacy of lower doses of afatinib on treatment outcomes in a real-world clinical practice. Data on clinical characteristics, afatinib dosing, and treatment outcomes for patients included in NCTSD from 1st January 2015 to 31st December 2020 were analyzed.
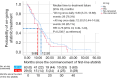
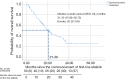
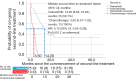
Results: Of the 133 patients studied, 94.7% had adenocarcinoma. Majority of the patients (60.9%) had EGFR exon 19 deletion and 23.3% had EGFR exon 21 L858R point mutation. The mean age of patients was 64.1 years and majority (83.5%) had Eastern Cooperative Oncology Group performance status of 2-4 at diagnosis. The most common afatinib starting doses were 40 mg (37.6%), 30 mg (29.3%), and 20 mg (26.3%) once daily (OD), respectively. A quarter of patients had dose reduction (23.3%) due to side effects or cost constraints. Majority of the patients had partial response to afatinib (63.2%) whilst 2.3% had complete response. Interestingly, the objective response rate was significantly higher (72.3%) with afatinib OD doses of less than 40 mg compared to 40 mg (54.0%) (P=0.032). Patients on lower doses of afatinib were two times more likely to achieve an objective response [odds ratio =2.64; 95% confidence interval (CI): 1.20-5.83; P=0.016]. These patients had a numerically but not statistically longer median time to treatment failure (TTF). Median TTF (95% CI) for the overall cohort was 12.4 (10.02-14.78) months. Median overall survival (95% CI) was 21.30 (15.86-26.75) months.
Conclusions: Lower afatinib doses (<40 mg OD) could be equally effective as standard dose in patients with EGFR-mutant advanced NSCLC and may be more suited to Asian patients, minimizing side effects that may occur at higher dosages of afatinib leading to dose interruptions and affecting treatment outcomes.
Keywords: Adenocarcinoma; resource-limited settings; survival; treatment outcome; tyrosine kinase inhibitors (TKIs).
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-691/coif). G.F.H. reports grants or contracts from AUXI Therapeutics and Taiho; payment or honoraria from AstraZeneca and Boehringer Ingelheim; support for attending meetings and/or travel from MSD; and participation on a Data Safety Monitoring Board or Advisory Board from Adipolab, Amgen, Astrazeneca, Boehringer Ingelheim, Dr Reddy, Eisai, MSD, Pfizer, Servier. Y.K.P. reports payments as speaker from AstraZeneca, GSK, and Pfizer; support for attending the European Respiratory Society Congress 2022 from EuroDrug; participation on Advisory Boards for Amgen, Eurodrug Laboratories, Boehringer Ingelheim, MSD, Novartis, Orient Europharma, Pfizer, Sanofi-Aventis, and Specialised Therapeutics; research funding from AstraZeneca, MSD, and Sanofi; duty as trustee for Lung Foundation of Malaysia, and role as the Immediate Past President for the Malaysian Thoracic Society. L.M.T. reports payment or honoraria from AstraZeneca, Boehringer Ingelheim, Fresenius Kabi, Janssen, Novartis, Pfizer, and Roche; and support for attending meetings and/or travel from AstraZeneca, Boehringer Ingelheim, Fresenius Kabi, and Roche. I.M.N. reports speaker honoraria from Eisei, MSD, Novartis, and Pfizer; Principal Investigator fees under Clinical Research Malaysia from Amgen, AstraZeneca, Mirati, MSD, and Novartis; travel grant for educational meetings/investigator meeting from Arcus, Eisei, ESMO, MSD, Novartis, and Roche; and monthly payments as Board of Director for Syarikat Hospital PUSRAWI Sdn. Bhd/ Syarikat MAIWP Healthcare Sdn Bhd from June 2021 to June 2023. K.F.H. reports payment or honoraria from EISAI; support for attending meetings and/or travel from Merck, and participation on a Data Safety Monitoring Board or Advisory Board for MSD. M.T. reports honoraria from Boehringer Ingelheim for a lecture in 2023 and is the current President of the Malaysian Oncological Society. S.H.H. reports funding from Boehringer Ingelheim for medical writing; grants or contracts from Arcus, AstraZeneca, Janssen, Merck, MSD, Novartis, and Pfizer; payment or honoraria from AstraZeneca, Janssen, MSD, Novartis, Pfizer, and Takeda; support for attending meetings and/or travel from MSD; participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, Janssen, MSD, Novartis, Pfizer, and Takeda, as well as receipt of equipment, materials, drugs, medical writing, gifts or other services from AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, and Takeda. The other authors have no conflicts of interest to declare.
Figures

References
-
- World Health Organization. Cancer [cited 2022 Feb 3]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer
-
- Globocan 2020. Malaysia [cited 2022 Mar]. Available online: https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-...
-
- Ministry of Health Malaysia. National Strategic Plan for Cancer Control Programme 2021-2025; 2021. Available online: https://www.moh.gov.my/moh/resources/Penerbitan/Rujukan/NCD/Kanser/Natio...
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
