Di- and Tricyanovinyl-Substituted Triphenylamines: Structural and Computational Studies
- PMID: 38496938
- PMCID: PMC10938432
- DOI: 10.1021/acsomega.3c05312
Di- and Tricyanovinyl-Substituted Triphenylamines: Structural and Computational Studies
Abstract
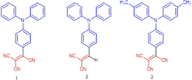
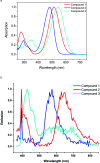
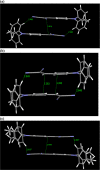
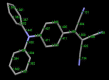
We report herein on the solid-state structures of three closely related triphenylamine derivatives endowed with tricyanovinyl (TCV) and dicyanovinyl (DCV) groups. The molecules described contain structural features commonly found in the design of functional organic materials, especially donor-acceptor molecular and polymeric architectures. The common feature noticeable in these structures is the impact of these exceptionally strong electron-accepting groups in forcing partial planarity of the portion of the molecule carrying these groups and directing the molecular packing in the solid state, resulting in the formation of π-stacks of dimers within the unit cell of each. Stacks are formed between phenyl groups bearing electron-accepting groups on two adjacent molecules. Short π-π stack distances ranging from 3.283 to 3.671 Å were observed. Such motif patterns are thought to be conducive for better charge transport in organic semiconductors and enhanced device performance. Intramolecular charge transfer is evident from the shortening of the observed experimental bond lengths in all three compounds. The nitrogen atoms (of the cyano groups) have been shown to be extensively involved in short contacts in all three structures, primarily through C-H···NC interactions with distances as short as 2.462 Å. The compounds reported here are (3,3-dicyano-2-(4-(diphenylamino)phenyl)-1λ3-allylidene)amide or tricyanovinyltriphenylamine, Ph3NTCV (1); 2-(4-(diphenylamino)benzylidene)-malononitrile or dicyanovinyltriphenylamine, Ph3NDCV (2); and (3,3-dicyano-2-(4-(di-p-tolylamino)phenyl)-1λ3-allylidene)amide or dimethyltricyanovinyltriphenylamine, Me2Ph3NTCV (3). Results of density functional theory calculations using DFT-B3LYP/6-31G(d,p) indicate the lowering of LUMO levels as a result of the introduction of these groups with band gaps of 3.13, 2.61, and 2.55 eV for compounds 1-3, respectively, compared with 4.65 eV calculated for triphenylamine. This is supported by the electronic and fluorescence spectra of these molecules with absorption λmax of 483, 515, and 545 nm for compounds 1, 2, and 3, respectively.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Crystal structure of 1-{4-[bis-(4-methyl-phen-yl)amino]-phen-yl}ethene-1,2,2-tricarbo-nitrile.Acta Crystallogr E Crystallogr Commun. 2024 Feb 29;80(Pt 3):339-342. doi: 10.1107/S2056989024001804. eCollection 2024 Mar 1. Acta Crystallogr E Crystallogr Commun. 2024. PMID: 38456047 Free PMC article.
-
Organic super-acceptors with efficient intramolecular charge-transfer interactions by [2+2] cycloadditions of TCNE, TCNQ, and F4-TCNQ to donor-substituted cyanoalkynes.Chemistry. 2009;15(16):4111-23. doi: 10.1002/chem.200802563. Chemistry. 2009. PMID: 19266523
-
Electron Donor and Acceptor Influence on the Nonlinear Optical Response of Diacetylene-Functionalized Organic Materials (DFOMs): Density Functional Theory Calculations.Molecules. 2019 Jun 2;24(11):2096. doi: 10.3390/molecules24112096. Molecules. 2019. PMID: 31159484 Free PMC article.
-
Solvent Effects on the Photophysical Properties of a Donor-acceptor Based Schiff Base.J Fluoresc. 2022 Jul;32(4):1321-1336. doi: 10.1007/s10895-022-02905-6. Epub 2022 Apr 2. J Fluoresc. 2022. PMID: 35366165
-
Experimental and theoretical study of donor-π-acceptor compounds based on malononitrile.Chem Cent J. 2018 Mar 9;12(1):26. doi: 10.1186/s13065-018-0394-5. Chem Cent J. 2018. PMID: 29524022 Free PMC article.
References
-
- Kong Q.; Qian H.; Zhou Y.; Li J.; Xiao H. Synthesis and Optoelectronic Properties of a Monodispersed Macrocycle Oligomer Consisting of Three Triarylamine Units. Mater. Chem. Phys. 2012, 135 (2), 1048–1056. 10.1016/j.matchemphys.2012.06.012. - DOI
-
-
See for example:
- Ogunyemi B. T.; Oyeneyin O. E.; Esan O. T.; Adejoro I. A. Computational Modelling and Characterization of Phosphole Adopted in Triphenyl Amine Photosensitizers for Solar Cell Applications. Results Chem. 2020, 2, 10006910.1016/j.rechem.2020.100069. - DOI
- El-Nahass M. M.; Zeyada H. M.; Abd-El-Rahman K. F.; Darwish A. A. A. Structural Characterization and Electrical Properties of Nanostructured 4-Tricyanovinyl-N,N-Diethylaniline Thin Films. Eur. Phys. J. – Appl. Phys. 2013, 62, 10202.10.1051/epjap/2013120061. - DOI
-
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
